Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

Phase 2 Trial of Voyager V1 in Combination With Cemiplimab in Cancer Patients
- Study Details
- Tabular View
- No Results Posted

- Age ≥18 years on day of signing informed consent.
Specific by tumor cohorts:
a. For the HSNCC cohort, histologically confirmed diagnosis of advanced and/or metastatic HSNCC suitable for first line immunotherapy.
i. HPV+ and HPV- patients are allowed.
ii. Primary tumor locations of oropharynx, oral cavity, hypopharynx, or larynx. Participants may not have a primary tumor site of nasopharynx (any histology).
iii. PD-L1 status ≥ 10% per local CPS score. Samples should be provided to central lab for post-hoc centralized testing.
iv. At least 12 months between last dose of prior adjuvant therapy and date of relapse diagnosis (if given). v. No prior anti-PD-(L)1 treatment for HNSCC.
b. For the melanoma cohorts, histologically confirmed diagnosis of advanced and/or metastatic cutaneous melanoma for which no existing options are considered to provide clinical benefit.
i. Best response of uPR, SD or PD to an anti-PD-(L)1-containing regimen.
ii. Prior anti-PD-(L)1 therapy must have lasted ≥ 12 weeks.
iii. Radiological progression was demonstrated during or after therapy with a PD-(L)1 immune CPI (only one prior line of PD-(L)1 therapy is permitted.
iv. If patient received anti-PD-1 as prior adjuvant therapy, patient should have relapsed during therapy or within the subsequent 6 months after last dose. Note: Progression on ipilimumab is not required.
v. Patients with BRAF V600-positive tumor(s) should have received prior treatment with a BRAF inhibitor (alone or in combination with a MEK inhibitor) in addition to treatment with an anti-PD-1 or to have declined targeted therapy. Note: Patients with BRAF V600-positive tumors with no clinically significant tumor-related symptoms nor evidence of rapidly progressive disease are not required to be treated with a BRAF inhibitor (alone or in combination with a MEK inhibitor) based on investigator's decision
c. For the CRC cohort, a histologically confirmed diagnosis of advanced and/or metastatic CRC.
i. Received or are not eligible for standard of care fluoropyrimidine(s), oxaliplatin, irinotecan, anti-VEGF and EGFR-targeted therapies.
ii. Non-microsatellite instability high (non-MSI high).
iii. Progression on previous systemic therapy.
- At least one tumor lesion amenable to IT injection and biopsy that has not been previously irradiated.
- Measurable disease based on RECIST 1.1., including ≥ 1 measurable lesion(s) to be injected
- Performance status of 0 or 1 on the ECOG Performance Scale
- Life expectancy of >3 months.
- Willingness to provide biological samples required for the duration of the study, including a fresh tumor biopsy sample whilst on study.
- Adequate organ function assessed by laboratory values obtained ≤14 days prior to enrollment
Patients meeting any of the following exclusion criteria at screening/Day -1 of first dosing will not be enrolled in the study:
- Availability of and patient acceptance of an alternative curative therapeutic option.
- Recent or ongoing serious infection, including any active Grade 3 or higher per the NCI CTCAE, v5.0 viral, bacterial, or fungal infection within 2 weeks of registration.
- Patients who have a diagnosis of ocular, mucosal or acral melanoma.
- Known seropositivity for and with active infection with HIV.
- Seropositive for and with evidence of active viral infection with HBV.
- Seropositive for and with active viral infection with HCV.
- Known history of active or latent TB.
- Any concomitant serious health condition, which, in the opinion of the investigator, would place the patient at undue risk from the study, including uncontrolled hypertension and/or diabetes, clinically significant pulmonary disease (e.g., chronic obstructive pulmonary disease requiring hospitalization within 3 months) or neurological disorder (e.g., seizure disorder active within 3 months).
Prior therapy within the following timeframe before the planned start of study treatment as follows:
- Small molecule inhibitors, and/or other investigational agent: ≤ 2 weeks or 5 half-lives, whichever is shorter.
- Chemotherapy, other monoclonal antibodies, antibody-drug conjugates, or other similar experimental therapies: ≤ 3 weeks or 5 half-lives, whichever is shorter.
- Radioimmunoconjugates or other similar experimental therapies ≤ 6 weeks or 5 half-lives, whichever is shorter.
- NYHA classification III or IV, known symptomatic coronary artery disease, or symptoms of coronary artery disease on systems review, or known cardiac arrhythmias (atrial fibrillation or SVT).
- Any known or suspected active organ-threatening autoimmune disease, such as inflammatory bowel disease, autoimmune hepatitis, lupus, or pneumonitis, with the exception of hypothyroidism and type 1 diabetes that are controlled with treatment
- Immunodeficiency or immunosuppression, including systemic corticosteroids at >10 mg/day prednisone or equivalent within 1 week prior to planned start of study treatment.
- Known concurrent malignancy.

- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 10, Issue Suppl 2
- 851 Voyager V1 (VV1) oncolytic virus combined with immune checkpoint therapy boosts CTL responses to multiple tumor antigens and correspondingly deepens tumor responses in murine models of melanoma, lung and colon cancer
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
https://doi.org/10.1136/jitc-2022-SITC2022.0851
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Read the full text or download the PDF:
- Clinical Trials
Phase 2 Trial of Voyager V1 in Combination With Cemiplimab in Cancer Patients
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
Study phase
During the early phases (phases 1 and 2), researchers assess safety, side effects, optimal dosages and risks/benefits. In the later phase (phase 3), researchers study whether the treatment works better than the current standard therapy. They also compare the safety of the new treatment with that of current treatments. Phase 3 trials include large numbers of people to make sure that the result is valid. There are also less common very early (phase 0) and later (phase 4) phases. Phase 0 trials are small trials that help researchers decide if a new agent should be tested in a phase 1 trial. Phase 4 trials look at long-term safety and effectiveness, after a new treatment has been approved and is on the market.
- Rochester, Minnesota: 20-000573
- Scottsdale/Phoenix, Arizona: 20-000573
- Jacksonville, Florida: 20-000573
About this study
The purpose of this study is to determine the preliminary anti-tumor activity and confirm the safety of VV1 in combination with Cemiplimab. The study will concurrently enroll patients with four distinct advanced malignancies in 5 separate tumor cohorts. The four cancer types are: Non-Small Cell Lung Cancer (NSCLC) and melanoma that are progressing on checkpoint inhibitor (CPI, generally refers to anti-PD(L)1 antibodies) treatment, CPI-naïve hepatocellular carcinoma (HCC), and treatment-naïve endometrioid endometrial cancer.
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Inclusion Criteria:
- Age ≥ 18 years on day of signing informed consent.
- No prior therapy with a PD-(L)1 immune checkpoint inhibitor (prior sorafenib is permitted);
- No or one prior line of systemic therapy only;
- Child Pugh Score A or B7.
- For the NSCLC cohort, histologically confirmed diagnosis of advanced and/or metastatic NSCLC in which radiological progression has been demonstrated during therapy with a PD(L)1 immune CPI and for which no existing options are felt to provide clinical benefit (only one line of PD-(L)1 therapy is permitted). Progression during or following 1 or more prior regimen(s) and no more than 3 prior therapeutic regimens for metastatic disease.
- At least one tumor lesion amenable to repeated IT injection via palpation or ultrasound. Injection of deep visceral lesions is not permitted;
- Agrees to provide a newly obtained biopsy of injected and witness lesions prior to start of study treatment, and to repeat biopsies twice during study treatment, and to providing the acquired tissue for biomarker analysis. Tissue obtained for the biopsy must not be previously irradiated, but a new or progressing lesion in the radiation field is acceptable.
- For the endometrial cancer cohort, histologically confirmed diagnosis of advanced and/or metastatic endometrioid endometrial adenocarcinoma. Eligible patients will not have had any prior systemic therapy in the metastatic setting.
- Last dose of anti-PD-(L)1 must be within 12 weeks of initiating study treatment.;
- Patient must have received at least 4 doses on q2w, 3 doses on q3w or 2 doses on q4w schedule of the previous anti-PD-(L)1 therapy;
- Documented radiographic progression on a single radiographic scan, if treatment with anti-PD-(L)1 was ≥ 16 weeks;
- Documented radiographic progression on two consecutive radiographic scans at least 4 weeks apart, if treatment with anti-PD-(L)1 therapy was between 8 - 16 weeks; if radiographic progression is accompanied with clinical progression, then a single scan assessment may be used;
- If progression was only in lymph nodes, biopsy to provide histological confirmation of progression in the lymph node is required.
- Measurable disease based on RECIST 1.1.
Exclusion Criteria:
Patients meeting any of the following exclusion criteria at Screening/Day-1 of first dosing will not be enrolled in the study.
- Availability of and patient acceptance of an alternative curative therapeutic option.
- Recent or ongoing serious infection, including any active Grade 3 or higher per the National Institute of Cancer Common Terminology Criteria for Adverse Events Version 5.0 (NCI CTCAE, v5.0) viral, bacterial, or fungal infection within 2 weeks of registration.
- Patients who are seropositive for HIV but are receiving antiviral therapy and show non-detectable viral load and a normal CD4 T cell count for at least 6 months are eligible;
- Seropositive for and with evidence of active viral infection with hepatitis B virus (HBV);
- Patients who are hepatitis B surface antigen (HBsAg) negative and HBV viral DNA negative are eligible;
- Patients who had HBV but have received an antiviral treatment and show non-detectable viral DNA for 6 months are eligible;
- Patients who are seropositive because of HBV vaccine are eligible.
- Patients who had HCV but have received an antiviral treatment and show no detectable HCV viral DNA for 6 months are eligible.
- Known history of active or latent TB (bacillus tuberculosis).
- Any concomitant serious health condition, which, in the opinion of the investigator, would place the patient at undue risk from the study, including uncontrolled hypertension and/or diabetes, clinically significant pulmonary disease (e.g., chronic obstructive pulmonary disease requiring hospitalization within 3 months) or neurological disorder (e.g., seizure disorder active within 3 months).
- Small molecule inhibitors, and/or other investigational agent: ≤ 2 weeks or 5 half-lives, whichever is shorter;
- ≤ 3 weeks or 5 half-lives, whichever is shorter;
- Antibody drug conjugates and radioimmunoconjugates or other similar experimental therapies ≤ 6 weeks or 5 half-lives, whichever is shorter.
- New York Heart Association (NYHA) classification III or IV, known symptomatic coronary artery disease, or symptoms of coronary artery disease on systems review, or known cardiac arrhythmias (atrial fibrillation or supraventricular tachycardia).
- Any known or suspected active organ-threatening autoimmune disease, such as inflammatory bowel disease, autoimmune hepatitis, lupus, or pneumonitis, with the exception of hypothyroidism and type 1 diabetes that are controlled with treatment .
- Immunodeficiency or immunosuppression.
- History of Grade 3 or 4 immune-mediated adverse reaction to immune CPIs.
- Toxicities from previous therapies that have not resolved to a Grade 1 or less.
- History of non-infectious pneumonitis that required steroids, or current pneumonitis.
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

Volume - 1999

Item added to your cart

Volume Bikes
- Voyager XL Frame
Couldn't load pickup availability
- Top Tube: 20.5″, 20.75″, 21″
- Chainstay: 13″ (13.25″ center to center)
- BB height: 11.7″
- BB Width: 74mm
- Seat Tube height: 9.25″
- Head Tube Angle: 75.25°
- Seat Tube Angle: 71°
- Seat Post: 25.4
- Headtube: Integrated (45°X45° Campy Spec)
- Brake Mounts: Removable Seatstay Mounts
- Dropouts: Heat Treated, 6mm thick with built in chain tensioners. Clears hub guards and pegs of all sizes.
- Colors: ED Black, Matte Trans Blue to Raw Fade(Jarren Barboza)
- Weight: 5 lbs 2 oz (21″)
The New Voyager XL frame features the same tech feel of the Voyager V2, but with a little extra length. It has a steeper 75.25º heat tube, Longer 13” slammed chainstay (13.25” center to center), a higher 9.25” seat tube height, and a 20.5” size for shorter riders. This frame also features double butted 4130 heat treated chromoly tubing, hour glass head tube, and small/ clean 6mm thick laser cut and welded dropouts with built in chain tensioner. Added top and bottom gussets for strength, removable brake mounts, and S chainstays for bigger tire clearance.
- Choosing a selection results in a full page refresh.
- Opens in a new window.
- Complete Wheels
- Inner Tubes & Rim Tapes
- Hub Parts/Spares
- Fork Top Caps
- Bottom Brackets
- Crank/BB Spares
- Seat Clamps
- Accessories
- Sweats & Hoods
- Animal Bikes
- Demolition Parts
- FIT Bike Co
- DVD's / Magazines
- Meet The Team
- My Account Login Register Log in
- BMX View All Bikes Frames Wheels Complete Wheels Hubs Rims Tyres Pegs Hub Guards Spokes Inner Tubes & Rim Tapes Hub Parts/Spares Steering Forks Bars Stems Headsets Grips Bar Ends Fork Top Caps Drive Train Bottom Brackets Chains Cranks Pedals Sprockets Crank/BB Spares Seating Seats Seat Posts Seat Clamps Braking Accessories Tools

Volume Voyager Frame
Volume’s Team Frame, the Voyager is based on specs from the whole volume bmx team, making this an ideal all round frame. Full Chro-Mo 4130 heat treated tubing featuring top and down tube gussets for strength, and hourglass head tube and S chain stays for bigger tyre clearance.
-Top Tube: 20.75”, 21”, 21.25" -BB Height: 11.7”. -BB Type: Mid. -Standover: 9.25". -Brakes: Removable. -Dropout size: 14mm. -Head Tube angle: 75.25°. -Headset type: Integrated. -Rear end length: 12.75”. (Slammed) -Seat Tube angle: 71°. -Colours: Trans Red, Gloss Black, Trans Blue -Weight: 5.2lbs.
HALF PRICE HEADSET & BB WITH ANY FRAME!
Simply add a frame, bottom bracket and headset to your basket and the website will do the rest 👌
You may also like
Customer reviews.

Get 20% OFF your next chain when you buy a new Sprocket from us. Discount is automatically added at the checkout!

Get 50% OFF a new BB & Headset for your new frame here at Foundation. Brand and Size is up to you. Discount added at the checkout!
July 28, 2018
Vyriad Announces Collaboration with Merck KGaA and Pfizer to Evaluate Oncolytic Virus, Voyager-V1, in Combination with Anti-PD-L1 Antibody, Avelumab, in Phase 1 Clinical Study for Metastatic Colorectal Cancer
ROCHESTER, Minn. – July 18, 2018 – Vyriad Inc., a clinical-stage, privately held biotechnology company focused on the development of powerful first-in class oncolytic virotherapies, is pleased to announce a collaboration agreement with Merck KGaA, Darmstadt, Germany, and Pfizer to expand its ongoing Phase 1 clinical trial program in solid tumors to include a combination study of its lead asset, Voyager-V1, with avelumab*, a human anti-PD-L1 antibody. For more information on this novel immuno-oncology combination study, please see clinicaltrials.gov .
“We are delighted to be working with Merck KGaA, Darmstadt Germany, and Pfizer on this innovative combination treatment approach,” said Stephen Russell, M.D., Ph.D., CEO of Vyriad. “Voyager-V1 is being administered to inflame the tumors, and avelumab has been shown to release the suppression of the T cellmediated antitumor immune response in preclinical models.”
“We are encouraged by the potential of Voyager-V1, which has demonstrated early clinical activity in patients with solid tumors,” said Alise Reicin, Head of Global Clinical Development at the Biopharma business of Merck KGaA, Darmstadt, Germany, which operates in the U.S. and Canada as EMD Serono. “We look forward to investigating how combining Voyager-V1 with avelumab may advance patient care.”
“A primary focus of our clinical development program for avelumab is to evaluate the role and potential of immunotherapy combination regimens, in an effort to support patients with challenging cancers,” said Chris Boshoff, M.D., Ph.D., Senior Vice President and Head of Immuno-Oncology, Early Development and Translational Oncology, Pfizer Global Product Development. “We look forward to working with Vyriad to explore this novel combination for patients with solid tumors.”
Avelumab has received accelerated approval** by the U.S. Food and Drug Administration (FDA) for the treatment of patients with metastatic Merkel cell carcinoma (MCC) and previously treated patients with locally advanced or metastatic urothelial carcinoma (mUC), and is under further clinical evaluation across a range of tumor types under a global strategic alliance between Merck KGaA, Darmstadt, Germany, and Pfizer.
*Avelumab is under clinical investigation for treatment of various solid tumors and has not been demonstrated to be safe and effective for this indication. There is no guarantee that avelumab will be approved for specific solid tumors by any health authority worldwide.
About Voyager-V1 Voyager-V1 (VSV-IFNβ-NIS) is derived from Vesicular Stomatitis Virus (VSV), a bullet-shaped, negativesense RNA virus with low human seroprevalence, specifically engineered to replicate selectively in and kill human cancer cells. Voyager-V1 encodes human IFNβ to boost antitumoral immune responses and increase tumor specificity, plus the thyroidal sodium iodide symporter NIS to allow imaging of virus spread. Three first-in-human Phase 1 clinical studies of Voyager-V1 are exploring intravenous and intratumoral routes of administration.
About Avelumab Avelumab is a human anti-programmed death ligand-1 (PD-L1) antibody. Avelumab has been shown in preclinical models to engage both the adaptive and innate immune functions. By blocking the interaction of PDL1 with PD-1 receptors, avelumab has been shown to release the suppression of the T cell-mediated antitumor immune response in preclinical models.1-3 Avelumab has also been shown to induce NK cell-mediated direct tumor cell lysis via antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro.3-5 In November 2014, Merck KGaA, Darmstadt, Germany, and Pfizer announced a strategic alliance to co-develop and cocommercialize avelumab.
Avelumab is currently being evaluated in the JAVELIN clinical development program, which involves at least 30 clinical programs, including seven Phase III trials and nearly 8,300 patients across more than 15 different tumor types. For a comprehensive list of all avelumab trials, please visit clinicaltrials.gov .
Indications in the U.S.** The U.S. Food and Drug Administration (FDA) granted accelerated approval for avelumab (BAVENCIO®) for the treatment of (i) adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma (mMCC) and (ii) patients with locally advanced or metastatic urothelial carcinoma (mUC) who have disease progression during or following platinum-containing chemotherapy, or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. These indications are approved under accelerated approval based on tumor response rate and duration of response. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Important Safety Information from the U.S. FDA-Approved Label The warnings and precautions for avelumab (BAVENCIO®) include immune-mediated adverse reactions (such as pneumonitis, hepatitis, colitis, endocrinopathies, nephritis and renal dysfunction and other adverse reactions), infusion-related reactions and embryo-fetal toxicity.
Common adverse reactions (reported in at least 20% of patients) in patients treated with BAVENCIO for mMCC and patients with locally advanced or metastatic UC include fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, peripheral edema, decreased appetite/hypophagia, urinary tract infection and rash.
For full prescribing information and medication guide for BAVENCIO, please see www.BAVENCIO.com .
Alliance between Merck KGaA, Darmstadt, Germany, and Pfizer Inc., New York, U.S. Immuno-oncology is a top priority for Merck KGaA, Darmstadt, Germany, and Pfizer Inc. The global strategic alliance between Merck KGaA, Darmstadt, Germany, and Pfizer Inc., New York, U.S., enables the companies to benefit from each other’s strengths and capabilities and further explore the therapeutic potential of avelumab, an anti-PD-L1 antibody initially discovered and developed by Merck KGaA, Darmstadt, Germany. The immunooncology alliance will jointly develop and commercialize avelumab and advance Pfizer’s PD-1 antibody. The alliance is focused on developing high-priority international clinical programs to investigate avelumab as a monotherapy, as well as in combination regimens, and is striving to find new ways to treat cancer.
About Vyriad Vyriad is a clinical-stage biotechnology company developing novel oncolytic virus therapies for the treatment of cancers that have significant unmet need. Vyriad’s oncolytic immunovirotherapy product candidates are based on the company’s engineered Oncolytic Vesicular Stomatitis Virus (VSV) and Oncolytic Measles Virus platforms that enable selective destruction of cancer cells without harming normal tissues.
Vyriad’s product development pipeline encompasses multiple clinical- and preclinical-stage programs that target a broad range of cancer indications, as well as programs that pair the company’s oncolytic viruses with other cancer immunotherapy modalities, traditional cancer therapy, and newer targeted therapies. Vyriad’s lead program, Voyager-V1, is in Phase 1 clinical research in solid tumors and hematological indications (please see clinicaltrials.gov). In addition, Vyriad is developing novel diagnostic/theranostic tests for more accurate prediction of immunovirotherapy response.
References 1. Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014;21(3):231-7. 2. Dahan R, Sega E, Engelhardt J et al. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 2015;28(3):285-95. 3. Boyerinas B, Jochems C, Fantini M et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015;3(10):1148-57. 4. Kohrt HE, Houot R, Marabelle A et al. Combination strategies to enhance antitumor ADCC. Immunotherapy 2012;4(5):511-27. 5. Hamilton G, Rath B. Avelumab: combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin Biol Ther 2017;17(4):515-23.
Media Inquiries
For inquiries, please contact [email protected]
Switch language:

Data Insights
Voyager-v1 by vyriad for endometrial cancer: likelihood of approval.

- Share on Linkedin
- Share on Facebook
Voyager-V1 is under clinical development by Vyriad and currently in Phase I for Endometrial Cancer. According to GlobalData, Phase I drugs for Endometrial Cancer have an 81% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how Voyager-V1’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks. Buy the report here.
Smarter leaders trust GlobalData
Premium insights likelihood of approval and phase transition success rate model - voyager-v1 in endometrial cancer.
Buy the Report
Premium Insights
The gold standard of business intelligence.
Find out more
Related Company Profiles
GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval.
Voyager-V1 overview
Voyager-V1 is under development for the treatment of metastatic melanoma and endometrial cancer and for relapsed and refractory hematological malignancies, solid tumors including metastatic colorectal cancer, pheochromocytoma, neuroendocrine tumor, endometrioid adenocarcinoma, non-small cell lung cancer, neuroendocrine carcinoma, serous adenocarcinoma, undifferentiated carcinoma, clear cell adenocarcinoma, mixed epithelial carcinoma, multiple myeloma, relapsed AML, non-Hodgkin lymphoma, T-cell lymphoma such as peripheral T-cell lymphoma, angioimmunoblastic T-cell lymphoma, anaplastic large cell lymphoma, myelodysplastic syndrome, cutaneous TCL (CTCL) of mycosis fungoides (MF). The therapeutic candidate is administered by the intravenous and intratumoral route of administration. The drug candidate, vesicular stomatitis virus (VSV)-interferon beta (IFNb)-sodium iodide symporter (NIS) is an oncolytic VSV encoding IFNb and the NIS reporter. The therapeutic candidate is developed based on VSV (VSV- IFNb-NIS) technology platform. The drug candidate acts by targeting interferon beta (IFNb) and SLC5A5.
It was also under development for metastatic hepatocullular carcinoma, breast, ovarian cancer, hepatocellular carcinoma and head and neck squamous cell carcinoma.
Vyriad overview
Vyriad is a clinical-stage biotechnology company that develops oncolytic virus therapies for the treatment of cancers. It develops drugs using two oncolytic virus platforms which include vesicular stomatitis virus (VSV) and measles virus (MV-NIS). The company’s pipeline products include Voyager-V1 + cemiplimab treats multiple cancers; Voyager-V1 + pembrolizumab for non-small cell lung cancer, head and neck cancer; MV-NIS monotherapy targets bladder cancer; Voyager-V1 monotherapy for solid tumors. Vyriad’s investigator-sponsored clinical studies include Voyager-V1 +/- ruxolitinib for multiple myeloma, T cell lymphoma; MV-NIS monotherapy targets medulloblastoma and recurrent atypical teratoid rhabdoid tumor (ATRT); Voyager-V1 +/- ruxolitinib for endometrial cancer. Vyriad is headquartered in Rochester, Minnesota, the US.
For a complete picture of Voyager-V1’s drug-specific PTSR and LoA scores, buy the report here.
This content was updated on 18 March 2024
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.
Be better informed
GlobalData , the leading provider of industry intelligence, provided the underlying data, research, and analysis used to produce this article.
GlobalData’s Likelihood of Approval analytics tool dynamically assesses and predicts how likely a drug will move to the next stage in clinical development (PTSR), as well as how likely the drug will be approved (LoA). This is based on a combination of machine learning and a proprietary algorithm to process data points from various databases found on GlobalData’s Pharmaceutical Intelligence Center .

Data Insights Likelihood of Approval and Phase Transition Success Rate Model - Voyager-V1 in Endometrial Cancer
More relevant.
EC approves Pfizer's Emblaveo for multidrug-resistant infections
Farletuzumab ecteribulin by eisai for ovarian cancer: likelihood of approval, alphamedix by orano med for neuroendocrine gastroenteropancreatic tumors (gep-net): likelihood of approval, lorcaserin hydrochloride by epygenix therapeutics for dravet syndrome (severe myoclonic epilepsy of infancy): likelihood of approval, sign up for our daily news round-up.
Give your business an edge with our leading industry insights.
Sign up to the newsletter: In Brief
Your corporate email address, i would also like to subscribe to:.
Pharma Technology Focus : Focus (monthly)
Thematic Take (monthly)
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.

Moscow City X

Price excl. VAT
Available as instant download
- Article number: AS13870
- Manufact./Publisher: Drzewiecki Design
- Language: English
- Current version: 1.6
Moscow City X is a very detailed model of Moscow metropolitan area in Russia, together with lite sceneries of 7 airports, many heliports and thousands of buildings. It took almost a year to complete and it includes countless fantastic features. The FPS/VAS-friendly design and advanced optimization techniques make the product's performance as satisfactory as possible.
Russia is the largest country in the world. It is a federal semi-presidential republic. From Northwest to Southeast, Russia shares land borders with Norway, Finland, Estonia, Latvia, Lithuania and Poland (both with Kaliningrad Oblast), Belarus, Ukraine, Georgia, Azerbaijan, Kazakhstan, China, Mongolia, and North Korea. Most of Russia consists of vast stretches of plains that are predominantly steppe to the south and heavily forested to the north, with tundra along the northern coast. Russia has about 1300 airports.
Moscow is the capital city and the most populous federal subject of Russia. The city is a major political, economic, cultural and scientific center in Russia and in Eastern Europe. According to Forbes 2013, Moscow has the largest number of billionaire residents in the world, has been ranked as the second most expensive city in the world by Mercer and is one of the world's largest urban economies, being ranked as an alpha global city according to the Globalization and World Cities Research Network and is also one of the fastest growing tourist destinations in the world according to the MasterCard Global Destination Cities Index. Moscow is the northernmost and coldest megacity and metropolis on Earth, the second most populous city in Europe after Istanbul and the 8th largest city proper in the world, as well as the largest amongst high income economies.
- Extremely detailed model of Moscow metropolitan area in Russia
- Almost 2000 custom-made buildings and other objects, all high quality, FPS-friendly and with night textures (some with bump/speculars)
- Whole Moscow center done in 3D as well as all other important landmarks - museums, palaces, skyscrapers, towers, bridges, railway stations, Zara stores...
- Trains, ships, 3D people, car traffic, animated vehicles, static aircraft - anything you can imagine
- About 4000 sq.km of seasonal/night photoreal terrain (50cm/pix in the center and 1m/pix the rest) with autogen and custom mesh
- Optional sceneries of all surrounding airports including UUWW Vnukovo, UUDD Domodedovo, UUBW Zhukovski, UUMO Ostafyevo, UUBM Myachkovo and UUMB Kubinka, with all airport buildings, detailed AFDs, 3D people, animated airport vehicles and more; each airport can be switched on/off as well as static aircraft at each airport
- HD asphalt and concrete taxiway/apron textures to use in the whole FS
- Very detailed Kremlin model with newly constructed heliport, some known individuals and animated clock on Spasska tower together with the most detailed Red Square flight sim model ever
- Red Square Landing Mission - a realistic recreation of M.Rust's 1987 landing with narration and special animations as well as customized default C172 in D-ECJB livery (with auxiliary fuel tanks)
Compatible with:
- UUEE Moscow Sheremetyevo X by Drzewiecki Design
- UUDD Moscow Domodedovo FSX/P3D by MDesign
- Do you have any questions concerning this product?
- Further products by Drzewiecki Design
Microsoft Flight Simulator X (incl. SP2 or Acceleration Pack, Gold Edition or Steam Edition) or Lockheed Martin - Prepar3D (V1-V5) Windows XP(SP2), Windows Vista, Windows 7, Windows 8 (64 bit recommended) 3 GHz processor (Dual Core processor or equivalent system required) 4 GB RAM Graphics card with al least 1024 MB Download-Size: 2 GB
Version 1.6:
- P3D V5 compatibility
Version 1.5:
- Installer fixes for P3D V4 installations
Version 1.4:
- Full compatibility with UUEE Moscow Sheremetyevo X V2 in terms of aerial image blendmask, autogen, mesh and car traffic
- UUBW - new static aircraft
- UUWW - new static aircraft, AFCAD fixes, new jetways, extra buildings added
- UUDD - new static aircraft, new airport layout, new terminal building, extra buildings added
Version 1.3:
- Seasons now match those in the simulator and in the UUEE Moscow Sheremetyevo X product
- FSW compatibility added
- GSX compatibility improved
- UUWW Vnukovo autogen fixes
- Highly upgraded installer script handling multiple AFD files better as well as mesh conflicts
You accept the following cookies by clicking on Accept all. You will find further information in the privacy settings, where you can also change your selection at any time. Just go to the page with the data protection declaration. View our data protection declaration.
Technically necessary cookies enable a website to store information already entered (such as user name or language selection) and to offer the user improved, more personalised functions.
share this!
April 23, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Tunable quantum anomalous Hall effects in van der Waals heterostructures
by Science China Press
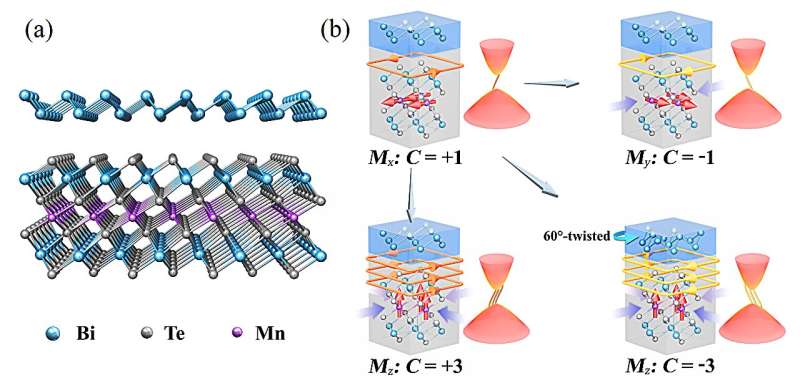
The quantum anomalous Hall effect (QAHE) has unique advantages in topotronic applications, but realizing the QAHE with tunable magnetic and topological properties for building functional devices is still a key scientific challenge. Through first-principles calculations, researchers have predicted a candidate material that meets these requirements.
The related work was recently published in the National Science Review under the title "Tunable quantum anomalous Hall effects in ferromagnetic van der Waals heterostructures."
Professors Wenhui Duan and Yong Xu from Tsinghua University's Department of Physics are the co-corresponding authors of the paper. Postdoc Feng Xue, affiliated with both the Department of Physics at Tsinghua University and the Beijing Academy of Quantum Information Sciences, is the first author.
Additional co-authors include Professor Ruqian Wu from the University of California, Irvine, Professor Ke He from Tsinghua University, Associate Professor Yusheng Hou from Sun Yat-sen University, doctoral student Zhe Wang from Fudan University, and doctoral student Qiming Xu from Tsinghua University.
The quantum anomalous Hall effect is a topological phenomenon characterized by the appearance of quantized Hall conductance without an external magnetic field , holding significant potential for next-generation electronic devices. Through systematic first-principles calculations, the research team predicts that QAHE induced by both in-plane and out-of-plane magnetization can be achieved within a single material system composed of van der Waals coupled Bi and MnBi 2 Te 4 monolayers.
By applying strain, magnetic field, or twisting the materials, significant changes in the magnetic and topological properties of the system can be induced, resulting in highly tunable QAHE states. This study not only provides a practical material platform for topological electronics but also opens new pathways for further experimental and theoretical exploration of the quantum anomalous Hall effect.
Provided by Science China Press
Explore further
Feedback to editors


Study shines light on properties and promise of hexagonal boron nitride, used in electronic and photonics technologies
9 minutes ago

Liquid droplets shape how cells respond to change, shows study
11 hours ago

Rice bran nanoparticles show promise as affordable and targeted anticancer agent

Advance in forensic fingerprint research provides new hope for cold cases

How spicy does mustard get depending on the soil?

Electron videography captures moving dance between proteins and lipids
12 hours ago

New findings shed light on how bella moths use poison to attract mates

AI tool creates 'synthetic' images of cells for enhanced microscopy analysis

Announcing the birth of QUIONE, a unique analog quantum processor

World's oases threatened by desertification, even as humans expand them
13 hours ago
Relevant PhysicsForums posts
Insulator band gap and applied voltage.
10 hours ago
Question about cgs vs SI units in the context of the Debye Length
16 hours ago
Looking for study group to hack on basic theory in condensed matter
Apr 20, 2024
Function to fit the light transmitted from a cavity
Apr 17, 2024
Hartree-Fock: Feynman diagrams vs perturbation theory
Cavity locking using lockin pid technique.
Apr 13, 2024
More from Atomic and Condensed Matter
Related Stories
Study unveils a new family of quantum anomalous Hall insulators
Apr 2, 2024

A balanced quantum Hall resistor provides a new measurement method
Apr 15, 2024
Reviewing the quantum anomalous Hall effect
Sep 15, 2020
Quantum question about the anomalous Hall effect answered
Aug 17, 2023

Experiments show that edges are not needed to realize an unusual quantum effect
Apr 18, 2023

Discoveries of high-Chern-number and high-temperature Chern insulator states
Jun 2, 2020
Recommended for you
New 2D material manipulates light with remarkable precision and minimal loss

Steering toward quantum simulation at scale
17 hours ago
Study shows ultra-thin two-dimensional materials can rotate the polarization of visible light

Superradiant atoms could push the boundaries of how precisely time can be measured

Bounding the amount of entanglement from witness operators
18 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Patients enrolled into three parallel doublet cohorts with an optimal Simon's two stage design. Patients will receive Voyager V1 as a direct to tumor injection (IT) in all 3 cancer groups and cemiplimab via IV infusion. Patients will return for treatment every 3 weeks until lack of clinical benefit or limiting toxicity.
Response is assessed at week 7 then Q9W per RECIST v1.1. The study includes serial biopsies in ≥3/10 pts in Stage 1 of each of the IV melanoma cohorts (doublet and triplet therapy), all pts in Stage 2 of these IV melanoma cohorts, and all pts in both Stage 1 and Stage 2 of the IV/IT melanoma cohort, to permit a thorough investigation of the ...
TPS3161 Background: VV1 is an oncolytic vesicular stomatitis virus engineered to express human IFNβ to enhance cellular anti-tumor immune responses and tumor selectivity, and the human sodium iodide symporter (NIS) for virus tracking by SPECT imaging. Cancer cells are often hyporesponsive to IFNβ, enabling the efficient spread of VV1 and resulting in increased oncolysis. Differently from ...
3090 Background: VV1 (Voyager V1) is derived from VSV, an RNA virus with low human seroprevalence, engineered to replicate selectively in and kill human cancer cells. In Part 1 of this study, we demonstrated the safety of intratumoral VV1 and dose-response, using serum IFNβ as a biomarker; we observed viral replication in tumor and concomitant lymphocyte/neutrophil trafficking (SITC 2018). 2 ...
Opens in a new window. Hand Powder Coated & painted by 503 BMX Voyager XL Frame Top Tube: 21″ or 20.75" Chainstay: 13″ (13.25″ center to center) BB height: 11.7″ BB Width: 74mm Seat Tube height: 9.25″ Head Tube Angle: 75.25° Seat Tube Angle: 71° Seat Post: 25.4 Headtube: Integrated (45°X45° Campy Spec) Brake Mounts: Removable ...
Voyager V-1 is our lead clinical asset based on the VSV platform. Voyager-V1 is designed for enhanced safety, efficacy, and traceability through the inclusion of the interferon beta gene, enabling the virus to: replicate quickly in cancer cells without damaging healthy cells. recruit cancer-fighting immune cells to the tumor, and.
Volume 10, Issue Suppl 2 ; 851 Voyager V1 (VV1) oncolytic virus combined with immune checkpoint therapy boosts CTL responses to multiple tumor antigens and correspondingly deepens tumor responses in murine models of melanoma, lung and colon cancer
The purpose of this study is to determine the preliminary anti-tumor activity and confirm the safety of VV1 in combination with Cemiplimab. The study will concurrently enroll patients with four distinct advanced malignancies in 5 separate tumor cohorts. The four cancer types are: Non-Small Cell Lung Cancer (NSCLC) and melanoma that are ...
Opdivo (nivolumab) • Yervoy (ipilimumab) • etoposide IV • Voyager-V1. Neoadjuvant systemic oncolytic vesicular stomatitis virus is safe and may enhance long-term survivorship in dogs with naturally occurring osteosarcoma. (PubMed, Mol Ther Oncolytics) An increase in tumor inflammation was observed in VSV-treated tumors and RNA-seq ...
The New Voyager XL frame features the same tech feel of the Voyager V2, but with a little extra length. It has a steeper 75.25º heat tube, Longer 13" slammed chainstay (13.25" center to center), a higher 9.25" seat tube height, and a 20.5" size for shorter riders.
Volume's Team Frame, the Voyager is based on specs from the whole volume bmx team, making this an ideal all round frame. Full Chro-Mo 4130 heat treated tubing featuring top and down tube gussets for strength, and hourglass head tube and S chain stays for bigger tyre clearance. -Top Tube: 20.75", 21", 21.25"-BB Height:
Phase II trial of Voyager-V1 (vesicular stomatitis virus expressing human IFNβ and NIS, VV1), in combination with cemiplimab (C) in patients with NSCLC, melanoma, HCC or endometrial carcinoma.
Voyager-V1 encodes human IFNβ to boost antitumoral immune responses and increase tumor specificity, plus the thyroidal sodium iodide symporter NIS to allow imaging of virus spread. Three first-in-human Phase 1 clinical studies of Voyager-V1 are exploring intravenous and intratumoral routes of administration.
According to GlobalData, Phase II drugs for Head And Neck Squamous Cell Carcinoma (HNSC) have a 26% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. GlobalData's report assesses how Voyager-V1's drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.
Voyager-V1 is under clinical development by Vyriad and currently in Phase II for Colorectal Cancer. According to GlobalData, Phase II drugs for Colorectal Cancer have a 25% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. GlobalData's report assesses how Voyager-V1's drug-specific PTSR and Likelihood ...
Voyager-V1 is under clinical development by Vyriad and currently in Phase I for Adenocarcinoma. According to GlobalData, Phase I drugs for Adenocarcinoma does not have sufficient historical data to build an indication benchmark PTSR for Phase I. GlobalData uses proprietary data and analytics to create drugs-specific PTSR and LoA in the Voyager-V1 LoA Report.
Voyager 1 stopped sending readable science and engineering data back to Earth on Nov. 14, ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
NASA's Voyager 1 resumes sending engineering updates to Earth. 11 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
NASA's Voyager 1 resumes sending engineering updates to Earth. 12 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
Trotsky's 1918-Volume 1, Military Writings Table of Contents. The Military Writings of. LEON TROTSKY. Volume 1, 1918 HOW THE REVOLUTION ARMED. These writings were first published in 1923 by the Soviet Government. They were translated by Brian Pearce. Annotation is by Brian Pearce.
Background: Voyager-V1 (VV1) is an oncolytic vesicular stomatitis virus engineered to express interferon beta (IFNβ) to boost cellular antitumor immunity and sodium iodine symporter (NIS). A phase 1 trial investigating a single infusion of VV1 in patients with relapsed refractory multiple myeloma and T-cell lymphoma (TCL) showed promising and durable clinical activity, primarily in patients ...
According to GlobalData, Phase I drugs for Endometrial Cancer have an 81% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData's report assesses how Voyager-V1's drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks. Buy the report here.
Voyager-V1 (VV1, VSV-IFNb-NIS) oncolytic virus in patients with relapsed refractory hematologic malignancies. KahWhyePeng,AdamRemick,NabilaNoraBennani,JoselleCook,LianwenZhang,ThomasE.Witzig,
Walking tour around Moscow-City.Thanks for watching!MY GEAR THAT I USEMinimalist Handheld SetupiPhone 11 128GB https://amzn.to/3zfqbboMic for Street https://...
NASA's Voyager 1 resumes sending engineering updates to Earth. 10 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
Map "Leninsky Prospekt, Moscow" v1.0 (0.31.x) Moscow Red Square v1.0 (0.30.X) Stanowice Region v1.67 (0.29.X) COMMENTS. Comments display order: 0 Spam. Andrey Krylov 17:05, 19.11.2023 . Клевый мод, всем советую! Автору спасибо) Log In: Write; Add modification. ADS. Social links ...
NASA's Voyager 1 resumes sending engineering updates to Earth. 10 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
Version 1.6: P3D V5 compatibility; Version 1.5: Installer fixes for P3D V4 installations; Version 1.4: Full compatibility with UUEE Moscow Sheremetyevo X V2 in terms of aerial image blendmask, autogen, mesh and car traffic; UUBW - new static aircraft; UUWW - new static aircraft, AFCAD fixes, new jetways, extra buildings added
NASA's Voyager 1 resumes sending engineering updates to Earth. 12 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story
NASA's Voyager 1 resumes sending engineering updates to Earth. 12 hours ago. ... However, we do not guarantee individual replies due to the high volume of messages. E-mail the story