Imperial Receives Recognition for Sustainability Performance! Learn more

Clinical Trial Enrollment Timelines: Hidden Causes Behind Big Delays

This is the second in my three-part series revealing hidden causes behind delays in clinical studies. The first part is here . This installment explores strategies to meet clinical trial enrollment goals.
Tell me when your first patient was enrolled, and I will tell you the chances your study will be a success. It’s not a magic trick, it’s just statistics.
First Patient In (FPI) is a key indicator of the overall success of a study. If that first patient is enrolled on time or within 30 days of the FPI goal, your study will begin on sound footing. The further you miss that goal, the more success will elude you. Here are the statistics:

Getting that first patient into the study on time is critical. If that goal isn’t met, it takes longer to gain traction and raises the likelihood that you will not hit your clinical trial enrollment targets. Your study will not meet its timelines.
This makes strategic patient recruitment an important tool in meeting FPI and LPFV (Last Patient/First Visit) goals.
In the past, about 70 percent of our business consisted of rescue programs for low-enrolling studies. We have seen considerable progress in this area: more than 70 percent of the studies we support today have proactive recruitment programs.
Sponsors have grown more sophisticated with recruitment, and many have even created internal departments tasked with accelerating enrollment. Merck’s Global Trial Optimization team is a noteworthy example.
Nevertheless, there is still room for improvement. A survey conducted by Imperial a few years ago revealed that 45 percent of line managers at sponsor companies had no proactive recruitment plans but also lacked the authority to put plans in place. Unfortunately, they are setting themselves up for delays.
Bring in the sites as a clinical trial enrollment strategy
Sites are the front lines of research and enrollment. Maximizing communication with sites early and often will help boost their performance.
Sites have tremendous insights and expertise that can be tapped. Here are tips on maximizing site performance:
- Capture site buy-in early and often
- Site support is a must-have topic at investigator meetings and training
- Involve clinical research coordinators in the planning and execution
- Arm sites with tools (75 percent of sites utilize patient recruitment materials)
- Show sites that you care and you are investing in their success and want their input
- Minimize the lag time between site feasibility, investigator meeting, and study initiation
Communicate on a fixed schedule

We find sites are vocal – and helpful — when asked for their perspective and input. We recommend forming site steering committees. These committees are made up of coordinators from top-performing sites and also mid and low-performing sites. Meet monthly or every six weeks to share recruitment and retention best practices as well as challenges.
And always remember that you are often competing with other sponsors’ studies for the site’s efforts and attention. Spending time with site staff and applying the tips I’ve mentioned to demonstrate your commitment can differentiate you from other sponsors and go a long way in motivating them to be enthusiastic about your study and clinical study enrollment goals.
Getting clinical trial enrollment right and getting it wrong
Studies that routinely meet FPI and LPFV timelines employ these planning strategies:
- Recruitment planning starts with protocol design
- Budgets are realistic
- Plans are revisited often
- Country startup planning ensures that the slowest locations are not activated last
- Outside voices (partner/recruitment vendor) are often utilized
- Physician referral programs
- Community outreach
- Advocacy groups
By contrast, here are common practices that will seal the fate of any study (yes, this really does happen):
- Not being competitive (lowballing contracts and grants)
- Paying sites late
- Falling into the key opinion leader trap (placing too much faith in “experts” or using the wrong experts)
- Failing to plan for backup sites to be activated if necessary
- Fixating on the latest single silver bullet, shiny ball approach
- Waiting too long to ask for help
Effective recruitment strategies, utilizing site expertise, and keeping a sharp focus on proactive planning are the heart of meeting clinical trial enrollment timelines and reaching study success.
Imperial is here to help. Optimize your clinical trial recruitment and retention plans with our evidence-based patient recommendations. Imperial’s diverse therapeutic knowledge and 25+ years of research patient engagement experience position us to collaborate with you on any indication and patient population. Our subject matter experts are here to help — contact us!
In my next post in this series, I will write about hidden delays in patient retention and compliance.
Dan McDonald
Dan is vice president, business development, Imperial Clinical Research Services. Dan is a seasoned executive who specializes in identifying income opportunities, building strategic partnerships, and managing contract negotiations. A prolific and popular thought leader, Dan has presented at numerous industry conferences and events, has conducted workshops and sessions on patient engagement, and has been published numerous times in industry books, trade magazines, and journals.
Printed Clinical Trial Materials: Planning for Investigator Meetings & Study Startup
Effective leadership: work on vs. work in the business, you may also like, clinical trial binders: are you doing it the..., initiatives to aid the clinical trials industry: a..., overcoming public skepticism in clinical trials, pending fda clinical trial diversity requirements, 6 top picks: clinical trial blogs not to..., 3 tips to avoid the holiday season impacting..., asking why bolsters clinical trial communication and success, clinical trials summit brings stakeholders to the table, the future of clinical trials, patient engagement, and..., strategies for retention in clinical trials: give thanks..., leave a comment cancel reply.
Switch language:

Operational Excellence in Clinical Trials
Ulrike Grimm, Vifor Pharma, analyses how clinical trial processes can be improved for the better
- Share on Linkedin
- Share on Facebook
Clinical trials have become a challenge. Few studies are completed on schedule and within the planned budget. There are plenty of reasons for that. In the past ten years:
Free Whitepaper
Unlocking the clinical trial potential of africa.

You will receive an email shortly. Please check for download the Whitepaper.
Go deeper with GlobalData

Clinical Trials - Real World Evidence
Intelligent treatments (healthcare) - thematic research, premium insights.
The gold standard of business intelligence.
Find out more
Related Company Profiles
Merck bio-pharma co ltd, ramp corporation, vifor pharma ag, fresenius biotech gmbh.
- The number of study procedures has increased by 57 percent translating into higher investigative site work
- The eligibility criteria have grown by 58 percent reflecting the search for always better defined patient populations
- The number of Case Report Form pages has more than doubled
Nevertheless, having said that, there is still the expectation the pharmaceutical industry executes trials according to plan bringing drugs to the market as soon as possible.
Thus the question to be asked is: What is wrong? The answer is not simple but rather complex.
It all begins with the evaluation of development compounds. The value of the assets is higher the shorter the development period, since this guarantees longer patent protection, potentially less competition and earlier generation of sales.
The development plans therefore often reflect best case scenarios and disregard the realistic case. Now we can ask ourselves, how do we come up with realistic plans for clinical studies?
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
I) There are several parameters that provide guidance during the preparation phase
1. Benchmarks
External benchmarks are available from several providers that have tracked past studies. In addition all studies conducted thus far in-house can be used to analyse the intervals between study milestones.
A combination of external and internal benchmarks to form a guide for studies in Europe and the US could look like this:
LPLV = Last Patient Last Visit
DBL = Data Base Lock
2. Recruitment period and recruitment plan
There are also benchmarks for recruitment periods per therapeutic area. However, I would caution to use high level, generic therapeutic area benchmarks for the recruitment period, because the study specific inclusion and exclusion criteria as well as the study procedure drive the recruitment period.
A detailed feasibility analysis should always be conducted to assess the recruitment period. Ideally this task is not fully outsourced to a CRO but also, or partially, done by the company’s own staff in order to have a detailed understanding of the availability of patients. The results of the feasibility analysis should be written down in a recruitment plan, where the following aspects are important to be considered:
- The availability of the required patient population at the sites;
- Implementation of referral sites;
- Competitive studies;
- Willingness of patients to participate in the study procedures;
- Advertisements;
- Patient retention;
- Risk assessment (what could go wrong?);
- Average recruitment speed of patients per months and site;
- Number of countries, proposed countries;
- Number of sites;
- Anticipated dates for the following milestones (1. First patient, First visit; 2. Last patient, First visit; 3. Last patient, Last visit; 4. Clinical study report)
3. Overall study plan
In addition to the scope, objectives, milestones and timelines, the overall study plan should include the study budget and the resource plan.
Regarding the study budget, it is usually the sum of all offers that have been received during the bidding process for the requested services. However, how many studies have been completed without any change orders? A certain amount of buffer is usually accepted by all internal stakeholders and should be added to the planned study budget.
The analysis of the resource needs is particularly important, because often major activities of the clinical study conduct are outsourced to a CRO and the need for internal resources can easily be underestimated.
It is recommended to check the internal resource needs against recently conducted studies to have a good understanding of the company-specific situation and approach.
4. CRO management
The CRO management starts with the CRO selection process. Usually a Request for Proposal is sent to several CROs and depending on the expertise, past experience in the therapeutic area, the team, and the budget a CRO will be selected. A good relationship between the study team of the sponsor and the CRO is of key importance. Therefore the bid defense meetings provide an excellent opportunity to bring together the envisioned teams of both parties.
In recent years we have seen high staff turnover at the CRO in some studies. Thus, it is advantageous to agree on replacement policies right from the beginning.
Moreover we have made good experience with agreeing on a communication plan that should detail the ways of communication and reports, and also the ways of escalation.
II) Tracking study progress during its conduct
As mentioned earlier, a risk analysis should be done during the preparation of the clinical study. Once the service providers have been selected, it is recommended to jointly work on a contingency plan.
Knowing that 20 – 30 percent of sites recruit only up to one patient, despite thorough feasibility assessments, really calls for back-up sites, may be even back-up countries early on. The triggers of activating these additional sites should be agreed with the steering committee.
Once the study has been initiated the progress will be monitored on an ongoing basis. Study dashboards allow for oversight and could look as shown below:
Dashboard of key performance indicators for clinical studies
As soon as deviations from the plan become obvious the study teams of the sponsor and the CRO should have meetings to discuss the best ways to mitigate the risks and bring the study back on track. Early communication is much better than a "wait and see" approach.
III) Lessons learned at the end of a study
Once the study report has been finalized and the TMF is ready for archiving, comes the time for lessons learned. It is worth to take the time and have a ‘lessons learned’ workshop, before the study team members will be allocated to other studies.
All parts of the study can be looked at and evaluated:
- What new benchmarks can be created from the study for timelines?
- What new benchmarks can be created for external costs and internal resource needs?
- How satisfied were we with the service providers?
- What countries are easy/difficult to work with?
- What sites would you work with again?
The outcome of these workshops should be made available on central places, e.g. on the intranet / central databases and managed by departments like clinical operations or clinical project management so that all study teams can benefit from it.
A thorough plan of all aspects of the clinical study will pay back in the end, by being prepared and able to pro-actively manage any risk and issues.
Close monitoring of the study progress will further enable the study teams to always be on top of any progress being made and to take action as soon as possible.
At the end of a study the ‘lessons learned’ workshops help to become better equipped for the planning of the next studies.
*Dr. Ulrike M. Grimm is the Head Global Project and R&D Alliance Management, Vice President of Vifor Pharma
Dr Grimm is responsible for Project and Alliance Management and joined Vifor Pharma in 2010. She has extensive leadership experience in R&D in oncology, CNS and Anemia, phase I – IV, international product launches, project & portfolio management and alliance management.
Before joining Vifor Pharma she was Vice President, Head of Global Program Management at Fresenius Biotech GmbH for three years.
Prior to this she was with Merck Serono for 10 years, in various roles of increasing responsibilities, including Global Product Leader, International Team Leader and Global Project Manager.
She is a Certified Projects Director at the highest IPMA Level A and Six Sigma Black Belt.
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
More Relevant
Forecasting tech key to tackling drug shortages in clinical trials, enlivex reports positive data from phase ii sepsis treatment trial, allay’s atx101 offers post-surgical pain relief in phase iib trial, acarix starts clinical workflow study for its ai-powered cardiac diagnostic, sign up to the newsletter: in brief, your corporate email address, i would also like to subscribe to:.
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
Advertisement
Protocol Design and Performance Benchmarks by Phase and by Oncology and Rare Disease Subgroups
- Original Research
- Published: 12 August 2022
- Volume 57 , pages 49–56, ( 2023 )
Cite this article
- Kenneth Getz ORCID: orcid.org/0000-0003-0568-2541 1 ,
- Zachary Smith 1 &
- Marcy Kravet 2
3748 Accesses
5 Citations
Explore all metrics
Benchmark data characterizing protocol design practices and performance informs clinical trial design decisions and serves as important baseline measures for assessing protocol design behaviors and their impact during and post-pandemic.
Tufts CSDD, in collaboration with a working group of 20 major and mid-sized pharmaceutical companies and CROs, gathered phase I–III data from protocols completed just prior to the start of the global pandemic.
Data for 187 protocols were analyzed to derive benchmarks overall and for two primary subgroups: oncology vs. non-oncology protocols and rare disease vs. non-rare disease protocols. The results show a continuing upward trend across all protocol design variables. Phase II and III protocols average more endpoints, eligibility criteria, protocol pages; investigative sites; countries and datapoints collected. Oncology and rare disease protocols’ enrolled-to-completion rates are much lower, involve a much higher average number of countries and investigative sites, require more planned patient visits and generate considerably more clinical research data. As such, oncology and rare disease clinical trial cycle times are longer—most notably at time periods occurring after study startup and prior to database lock—due to intense patient recruitment and retention challenges.
Conclusions
The results of this study present valuable design insights and comparative baseline measures. The implications of these results and the expected impact of decentralized clinical trials on protocol design practices and performance is discussed.
Similar content being viewed by others

New Benchmarks on Protocol Amendment Experience in Oncology Clinical Trials
Emily Botto, Zachary Smith & Kenneth Getz
Benchmarking Protocol Deviations and Their Variation by Major Disease Categories
Kenneth Getz, Zachary Smith, … Randy Krauss
New Benchmarks on Protocol Amendment Practices, Trends and their Impact on Clinical Trial Performance
Kenneth Getz, Zachary Smith, … Arnaud Dauchy
Avoid common mistakes on your manuscript.
Introduction
The drivers of protocol complexity are constantly evolving in step with the strategies that dominate drug development at any given time. In the 1980s, for example, the pursuit of blockbuster therapies expanded the number of assessments conducted, clinical investigators engaged and patients enrolled in later stage, phase III clinical trial designs [ 1 ]. During the next decade, cost containment measures and growing interest in cycle time reduction prompted clinical teams to increase their use of contract research organizations (CROs) and engage with larger numbers of private sector, community-based investigative sites [ 2 , 3 ]. During this decade, protocol designs began capturing more endpoints and conducting even more assessments—most notably in phase II—in an effort to inform, and even avoid, transitioning into the more expensive phase III clinical trials [ 4 ].
Pressure to reach treatment-naïve patient communities, identify less expensive though well-trained investigators, and support simultaneous international submissions drove more globally oriented protocol designs in the early 2000s [ 5 , 6 ]. During this decade, regulatory agency interest in quality by design principles and in improving risk evaluation and mitigation drove growth in the number of safety procedures and the volume of data collected in phase I and II protocol designs [ 7 , 8 , 9 ].
Between 2010 and 2020, sponsor companies, in pursuit of more flexible and efficient clinical trials, piloted and implemented more novel designs—including adaptive and master protocols [ 10 , 11 , 12 ]. During this period, the proportion of programs in the global drug development pipeline targeting rare diseases and narrowly defined patient subpopulations increased dramatically supported by rapid growth in the volume of biomarker and genetic data collected per protocol [ 13 ]. The end of this decade also saw heightened interest in collecting real-world data and patient health information to supplement, and even replace, data collected during the clinical trial [ 14 ].
Since the 1980s, nearly all protocol design changes—both scientific (e.g., number of endpoints, eligibility criteria and procedures performed) and executional (e.g., number of countries and Investigative sites)—have been additive. In the past two decades, research routinely and periodically conducted by the Tufts Center for the Study of Drug Development (Tufts CSDD) in collaboration with several dozen pharmaceutical companies finds that benchmarked protocol designs have yet to show a downward trend in any given design element [ 15 , 16 ].
Tufts CSDD research also demonstrates that as protocol designs become larger in scope and more demanding, clinical trial performance worsens. Protocols with a higher relative number of endpoints, eligibility criteria and procedures are associated with lower physician referral rates; increased procedure administration burden; diminished study volunteer willingness to participate; lower patient recruitment and retention rates; lower dose adherence; increased data volume; and a higher incidence of protocol deviations and substantial amendments. Ultimately, these outcomes contribute to higher failure rates, longer clinical trial cycle times, poorer data quality and greater drug development study and program costs [ 17 , 18 ].
Early in the current decade (2020–2030) the rapid deployment and adoption of decentralized clinical trials (DCT) has already been recognized as an important and defining new drug development strategy. Virtual and remote approaches include the use of telemedicine, wearable devices; mobile applications; procedures performed at more convenient locations by visiting study staff; and investigational drugs delivered directly to the study volunteer’s home. The shift to decentralized clinical trials has been facilitated largely by the COVID-19 pandemic and by heightened interest in improving access to, engaging and enrolling more, demographically diverse study volunteers [ 19 ].
Empirical data characterizing the impact of DCTs on protocol design are yet to be collected. This paper presents the results of a study benchmarking protocol design practice just before the onset of the global, COVID-19 pandemic. As such, it provides a valuable opportunity to serve as an important baseline for making comparisons and drawing insights on ways to optimize protocol designs developed and executed during and post-pandemic. This paper also presents data providing comparisons between two primary subgroups—oncology vs. non-oncology and rare disease vs. non-rare diseases. These subgroups are the most active areas in the drug development pipeline and they receive the most frequent requests for benchmarks by sponsor companies.
Clinical and clinical operations professionals from 20 major and mid-sized pharmaceutical companies and CROs—Amgen, AstraZeneca, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL Behring, Eli Lilly, EMD Serono, GlaxoSmithKline, Janssen, Merck, Novartis, Otsuka, Parexel, Pfizer, Roche, Sanofi, Takeda, UCB, Veristat—provided protocol design and performance data.
Each company was asked to select protocols representative of their current portfolio of clinical trial activity and to include protocols from each of three phases (i.e., Phase I, Phase II, and Phase III). The convenience sampling frame included only those protocols that had received final protocol approval between January 2013 and December 2018 and had a primary completion date or database lock date prior to December 31st, 2019. CROs participating in the study gathered protocol data specifically from client companies other than those represented by sponsor companies in the working group. On average, each participating company submitted data characterizing 11 protocols.
The data collection process used in this study is consistent with the methodology that Tufts CSDD has been using since 2008 to evaluate protocol design practices and their impact. The results of these studies have been published extensively. In each of these studies, design variables typically gathered include the number and type of endpoints, number of eligibility criteria, number of distinct and total procedures performed, number of countries and investigative sites where the protocol was conducted, and number of planned study volunteer visits per month.
Clinical trial performance and quality variables typically gathered by Tufts CSDD include clinical trial milestone durations, recruitment and retention rates. Performance and quality variable definitions are as follows:
Study Initiation Duration—days from Protocol Approval to First Patient First Visit (FPFV);
Enrollment Duration—days from First Patient First Visit (FPFV) to Last Patient First Visit (LPFV);
Treatment Duration—days from Last Patient First Visit (LPFV) to Last Patient Last Visit (LPLV);
Study Close-out Duration—days from Last Patient Last Visit (LPLV) to Database Lock (DBL);
Total Clinical Trial Duration—days from Protocol Approval to Database Lock (DBL);
Patient Randomization Rate—the ratio of the number of patients enrolled to the total number screened;
Patient Completion Rate—the ratio of the number of patients completing the clinical trial to the total number enrolled.
Participating companies also classified each protocol procedure according to the endpoint that it supported as defined by the clinical study report and the study’s statistical analysis plan. ‘Core’ procedures supported primary and key secondary efficacy and safety endpoints. ‘Non-Core’ procedures supported supplemental secondary, tertiary and exploratory safety, efficacy or other endpoints and objectives.
The analysis dataset excluded master protocols and adaptive designs to focus on only traditional protocol design practices. We combined data for Phase II and III protocols for comparisons by therapeutic area, by oncology vs. non-oncology, and rare disease vs. non-rare disease, given the smaller sample sizes by individual phase. Descriptive statistics including means and coefficients of variation were calculated. The latter measure is an indication of the consistency in experience between and across participating companies. Protocol data were stored as an excel file and saved on a secure, shared, online drive. The analysis was conducted in SAS 9.4.
In all, 187 protocols were analyzed. Table 1 presents characteristics of the analysis dataset. It contains similar numbers of Phase II (72) and Phase III (67) protocols, with somewhat fewer Phase I (48) protocols. Slightly more than a quarter of the protocols (27.3%) targeted oncology diseases and approximately 1 in 5 protocols (17.7%) targeted rare disease indications.
Table 2 provides means for several scientific design characteristics by phase. Generally, these characteristics are lowest for Phase I protocols. Phase II protocols have the highest mean number of endpoints (20.7). Phase III protocols have the highest mean number of distinct (34.5) and total procedures (266.0) and total protocol pages (115.9). The mean number of datapoints collected per protocol by phase shows a strong progression from 330,420 in phase I, to 2,091,577 in phase II, and 3,453,133 in Phase III. The coefficients of variation around the mean scientific design characteristics are generally very high, most notably the total procedures performed, proportion of procedures that are non-core, total case report form pages and total datapoints collected. A significant correlation was observed between the number of endpoints and the number of eligibility criteria ( p < 0.01) and the number of endpoints and the total number of datapoints collected ( p < 0.05).
The means for scientific design characteristics—phase II and III combined—are presented for oncology vs. non-oncology protocols in Table 3 . The means and coefficients of variation for many design characteristics are comparable between oncology and non-oncology protocols including the mean number of eligibility criteria (29.8 and 31.0), the mean number of distinct procedures (33.3 and 34.3), the average proportion of procedures that are non-core (24.1% and 24.9%) and the mean number of total datapoints collected (2.6 million and 2.7 million). The mean number of total procedures performed was substantially higher for oncology vs. non-oncology protocols at 315 and 243, respectively. Non-oncology protocols have a higher mean number of endpoints (21.4 vs. 15.3 for oncology protocols). No significant relationship was observed between the number of endpoints, the number of eligibility criteria and the total number of datapoints collected in oncology protocols.
Table 3 also shows notable differences observed between protocols targeting rare vs. non-rare diseases with the latter having much higher mean total number of endpoints (12.9 for rare disease and 21.2 for non-rare disease protocols); average proportion of non-core procedures (14.0% for rare disease and 26.4% for non-rare disease protocols); and mean total number of datapoints collected (1.6 million for rare disease and 2.9 million for non-rare disease protocols). Non-rare disease protocols collect nearly double the amount of data than do rare disease protocols.
Rare disease protocols have a higher mean number of distinct procedures (38.1 for rare disease and 33.3 for non-rare disease protocols), mean total number of procedures performed (301.6 for rare disease and 255.6 for non-rare disease protocols), and mean number of case report form pages (244.0 for rare disease and 158.7 for non-rare disease protocols). A significant correlation was observed between the number of endpoints and the total number of datapoints collected ( p < 0.01) in rare disease protocols.
Means for executional design characteristics per protocol, by phase, are presented in Table 4 . These characteristics include the mean total number of countries, mean total number of planned visits, and the mean total number of patients screened and enrolled. The typical phase III protocol, for example, has more than double the average number of countries and investigative sites than does the typical phase II protocol. Very high coefficients of variation are observed around the mean values for most executional variables, in particular the mean number of investigative sites, number of patients screened, enrolled and completing clinical trials by phase.
Table 5 presents the executional design characteristics by oncology and rare disease subgroups. With few exceptions, oncology protocols have higher mean executional variable values than do non-oncology protocols including the average number of countries, investigative sites, planned visits. Exceptions include the mean number of vendors (4.4 for oncology and 5.8 for non-oncology, mean number of procedures per visit (11.9 for oncology and 14.4 for non-oncology protocols) and the mean number of patients completing clinical trials (244.9 for oncology and 291.1 for non-oncology protocols). Among oncology protocols, the coefficient of variation is very high around the mean number of patients completing the clinical trial indicating widely varied experiences between studies and sponsors.
Many mean values for executional design characteristics are similar between rare disease and non-rare disease protocols. Exceptions include the mean number of investigative sites, mean number of patients screened, enrolled and completing clinical trials where the benchmark values for non-rare disease protocols are considerably higher. The mean number of planned visits and days for follow-up are higher for rare disease compared to none-rare disease protocols. The coefficients of variation for both rare disease and non-rare disease protocols are generally very high–in particular those associated with patient recruitment and retention.
Tables 6 and 7 contain benchmarks for select protocol performance outcomes. In Table 6 , mean performance outcomes are shown per protocol by phase. The mean treatment duration for a phase III protocol is 2.2 times longer than the typical phase I, and 1.3 times longer than the typical phase II, protocol. The average total clinical trial duration—from protocol finalization to database lock—for a phase III protocol is approximately 1,328 days.
Mean durations are longer for later stage protocols with two exceptions: study close-out duration and time to clinical study report. Protocol randomization and completion rates are also similar between phases, although the completion rate for Phase I trials was slightly higher than that observed in phase II and III protocols.
Oncology protocols show longer cycle time durations than do non-oncology protocols for all clinical trial durations except study initiation (see Table 7 ). On average, phase II/III oncology protocols are 1.5 times longer than non-oncology protocols with the widest differences observed in durations associated with patient enrollment. Completion rate was also substantially lower for oncology protocols than for non-oncology protocols—31.4% and 80.0%, respectively. Protocols targeting rare diseases have longer cycle time durations for most measures except study conduct, study close-out, and time to clinical study report. Protocols targeting rare disease also had lower completion rates than did non-rare disease protocols—50.8% and 72.5%, respectively. The most notable difference in clinical trial durations is observed in the time to complete each enrolled patients’ first visit.
Table 8 shows trends in select scientific and executional design characteristics. Mean values per protocol, in phase II and III, are presented in four-year increments between 2009 and 2020. An upward trend is observed for all variables. The total mean number of countries and the total mean number of procedures performed showed the highest relative growth rates during this period with both increasing by slightly less than 70% over the time horizon measured. Others design variables showed more moderate but still substantial growth including mean total number of investigative sites, which increased by 33.0%, and mean total number of endpoints, which increased by 27.1%.
The results of this study provide data that can serve as benchmarks for proactively assessing the scientific and executional complexity of new protocols. These benchmarks also establish important baselines for measuring the impact of the pandemic on future protocol design practices.
The results show a continuing upward trend across all protocol design variables. Phase II and III protocols now average 20.7 and 18.6 total endpoints, respectively; 30.9 and 30.4 inclusion and exclusion criteria; 107.6 and 115.9 protocol pages; 35.1 and 82.2 investigative sites disbursed within 6.1 and 13.7 countries, respectively; and 2.1 million and 3.5 million datapoints collected, respectively.
These findings are an expected consequence of increasingly more ambitious and customized drug development strategies driven in part by highly challenging disease targets in active R&D; strong demand for data to understand differences between patient subgroups (e.g., biomarker stratification); and great difficulty associated with identifying, competing for, recruiting and retaining study sites and volunteers.
Whereas oncology and rare disease protocols have average numbers of endpoints and eligibility criteria comparable to non-oncology and non-rare disease protocols, wide differences are observed in the executional variables. Although oncology and rare disease protocols have considerably lower relative target patient enrollment numbers, they involve a much higher average number of countries and investigative sites, require more patient visits per protocol and generate considerably more clinical research data that must be monitored, cleaned, curated and analyzed.
Oncology and rare disease clinical trial durations are longer—most notably between study startup and database lock. This is due in part to the long follow-up periods found in oncology and rare disease studies: the former had a mean days-for-follow-up four times longer than that observed in non-oncology protocols; and the latter rare disease protocols had a mean-days-for-follow-up nearly 2.5 times longer than the comparison non-rare group. In our dataset, more than 80% of oncology protocols had completion times that were event-driven as opposed to fixed-duration driven. This compares to non-oncology protocols where only 9% had event-driven completion times. Further, completion metrics for oncology clinical trials may have been substantially longer due, in part, to disease progression leading to early discontinuation. Rare disease protocols also had longer relative study initiation periods likely due to the difficulty in engaging investigative sites and in finding and enrolling study volunteers.
The results of this study, combined with those from a recent Tufts CSDD study looking at design variables correlated with clinical trial performance [ 18 ], also suggest practical considerations for protocol design decision-makers. Strong observed growth in the number of investigative sites and countries supporting protocol execution–and the significant positive correlation between these executional design variables and clinical trial durations–represents a substantial opportunity to improve speed and efficiency. The relatively high proportion of non-core procedures, most notably in non-oncology and non-rare disease protocols, suggests a critical need and opportunity to reduce and simplify the total number of less essential endpoints and the protocol procedures supporting them.
This study has several limitations of note: The protocols were selected by participating companies arbitrarily and, as such, represent a convenience sample. Moreover, the benchmarks are based on aggregated data drawn from a wide variety of disease conditions. The large coefficients of variation observed around the mean values indicate that the benchmarks should be used with some caution.
Future research will look to gather a larger sample of protocols so that comparisons by individual disease conditions can be made. Tufts CSDD is also planning to explore the relationship between protocol complexity and the ethics review cycle, the regulatory review and approval cycle and its outcome, and between protocol complexity and commercialization performance.
As drug development strategies evolve and decentralized clinical trial solutions gain acceptance, we can expect to see ongoing changes in protocol designs. Data volume and data diversity, for example, will likely increase with more widespread adoption of handheld devices and mobile apps and greater integration of patient health data into the clinical trial analysis dataset.
As clinical trials for select disease conditions move to wherever and whenever patients can most easily and conveniently participate, we may see more countries involved in clinical trials but fewer physical investigative site locations. Early anecdotal reports suggest that DCTs may shorten clinical trial durations through faster recruitment and better retention and a reduction in the number of protocol amendments. Some anecdotal reports also suggest that the introduction of new DCT vendors, non-standard datasets, training requirements and novel practices, at least in the short term, may contribute to higher levels of protocol complexity.
As these changes unfold, we look forward to continuing our research benchmarking protocol design behaviors and their impact on clinical trial performance.
Sampler S. Tracking protocol complexity. GCPJ. 2000;7(2):6–8.
Google Scholar
Vogel J, Getz K. Factors driving the increases use of contractors in drug development. Clin Res Reg Affairs. 1997;14(4):177–90.
Article Google Scholar
Maloy J, Getz K, Hovde M. Clinical research in transition. Monitor. 2001;15:31–6.
Kahn M, Broverman C, Wu N, Farnsworth W, Manlapaz-Espiritu L. Improving protocol quality. Appl Clin Trials. 2002;11:40–50.
Rai S. Drug companies cut costs with foreign clinical trials. The New York Times. 2005. http://www.nytimes.com/2005/02/24/business/24clinic.html?8bl .
Getz K, Brown C, Stergiopoulos S, Beltre C. Baseline assessment of a global clinical investigator landscape poised for structural change. TIRS. 2017;51(5):575–81.
Woodcock J. The concept of pharmaceutical quality. Am Pharm Rev. 2004;1:1–3.
Rathore A, Winkle H. Quality by design for biopharmaceuticals. Nat Biotechnol. 2009;27:26–34.
Article CAS Google Scholar
Getz K. Protocol design trends and their effect on clinical trial performance. RegAffairs. 2008;19:315–6.
Bhatt D, Mehta C. Adaptive designs for clinical trials. NEJM. 2016;375:65–74.
Pallmann P, Bedding A, Choodari-Oskooei B, Dimairo M, Flight L, Hampson L, Holmes J, Mander A, Odondi L, Sydes M, Villar S, Wason J, Weir C, Wheeler G, Yap C, Jaki T. Adaptive designs in clinical trials: why use them, and how to run them. BMC Med. 2018;16(29):2–15.
Meyer E, Mesenbrink P, Dunger-Baldauf C, Fülle H, Glimm E, Li Y, Posch M, Konig F. The evolution of master protocol clinical trial designs. ClinTher. 2020;42(7):1330–60.
Getz K, Campo R. New benchmarks characterizing growth in protocol design complexity. TIRS. 2018;52:22–8.
Lamberti M, Kubick W, Awatin J, McCormick J, Carrol J, Getz K. The use of real world evidence and data in clinical research band post-approval safety studies. TIRS. 2018;52:778–83.
Getz K. Improving protocol design feasibility to drive drug development economics and performance. Int J Environ Res Public Health. 2014;11:5069–80.
Getz K, Campo RA. New benchmarks characterizing growth in protocol design complexity. TIRS. 2018;52(1):22–8.
Getz K, Stergiopoulos S, Short M, Surgeon L, Krauss R, Pretorius S, Desmond J, Dunn D. The impact of protocol amendments on clinical trial performance and cost. TIRS. 2016;50(4):436–41.
Smith Z, Bilke R, Pretorius S, Getz K. Protocol design variables highly correlated with, and predictive of clinical trial performance. TIRS. 2021. https://doi.org/10.1007/s43441-021-00370-0 .
Apostolates M, Barbadian D, Cornelli A, Forrest A, Hamre G, Hewitt J, Podolsky L, Popat V, Randall P. Legal, regulatory and practical issues to consider when adopting decentralized clinical trials. TIRS. 2020;54:779–87.
Download references
Acknowledgments
Thank you to the many companies that participated and supported this working group study: Amgen, AstraZeneca, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL Behring, Eli Lilly, EMD Serono, GlaxoSmithKline, Janssen, Merck, Novartis, Otsuka, Parexel, Pfizer, Roche, Sanofi, Takeda, UCB, Veristat. The authors also wish to thank Michael Wilkinson for his contributions to this research.
Tufts CSDD received grant funding from the participating working group companies to cover staff time on this study.
Author information
Authors and affiliations.
Tufts Center for the Study of Drug Development, Tufts University School of Medicine, 145 Harrison Avenue, Boston, MA, 02111, USA
Kenneth Getz & Zachary Smith
EMD Serono, Rockland, MA, USA
Marcy Kravet
You can also search for this author in PubMed Google Scholar
Contributions
KG, TCSDD, contributed to all four aspects (substantial contribution to conception, design, analysis, interpretation; drafting and revising the work; final approval of the version to be published; agreement to be accountable for all aspects in ensuring accuracy and integrity of the work). ZS, TCSDD, contributed to all four aspects; MK, EMD Serono, made substantial contribution to conception, design, analysis and interpretation.
Corresponding author
Correspondence to Kenneth Getz .
Ethics declarations
Conflict of interest.
Kenneth Getz, Tufts CSDD and Zachary Smith, Tufts CSDD have nothing to disclose. Marcy Kravet, EMD Serono, declares that she is an employee and has financial holdings in the company.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Getz, K., Smith, Z. & Kravet, M. Protocol Design and Performance Benchmarks by Phase and by Oncology and Rare Disease Subgroups. Ther Innov Regul Sci 57 , 49–56 (2023). https://doi.org/10.1007/s43441-022-00438-5
Download citation
Received : 31 January 2022
Accepted : 20 July 2022
Published : 12 August 2022
Issue Date : January 2023
DOI : https://doi.org/10.1007/s43441-022-00438-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Protocol design
- Protocol complexity
- Oncology protocols
- Rare disease protocols
- Protocol scope
- Clinical trial performance benchmarks
- Find a journal
- Publish with us
- Track your research

Last patient last visit (LPLV)
Date when the last participant completes a clinical trial .
Although LPLV is often used synonymously with study completion, the latter may still include measurements and data collection without face-to-face involvement between a participant and an investigator.
The Clinical Trial Life Cycle and When to Share Data (Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risks, 2015)
Subscribe to our newsletter to stay up-to-date about our webinars, conferences and other activities.
Personal information - Select salutation - Mrs Mr Mx Dr Prof
First name *
Last name *
Organization
Desired content General Communications Scientific Affairs & REVIVE
I accept the terms of use and privacy notice
View previous newsletter
Imperial College London Imperial College London
Latest news.

Superfast physics and a trio of Fellows: News from Imperial

'Living paint’ startup wins Imperial’s top entrepreneurship prize

Imperial wins University Challenge for historic fifth time
- Joint Research Office
- Research and Innovation
- Support for staff
- Healthcare research studies
- Recruitment stage
Last Patient Last Visit
Last Patient Last Visit defines the date that the last subject completed the study.
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Quantifying Protocol Deviation Experience by Clinical Phase
Study uncovers pre-pandemic deviation levels.


A baseline assessment
To our knowledge, little-to-no data exists quantifying and benchmarking protocol deviation experience overall and by clinical phase. For this reason, Tufts CSDD engaged a working group of 20 major and mid-sized pharmaceutical companies and contract research organizations (CROs) to gather hard data on deviations associated with traditional clinical trial protocols (i.e., master protocols and adaptive designs were not included). Our study sample included protocols that had received final approval between January 2013 and December 2018 and had a primary completion date or database lock date before December 31, 2019. The data-collection process deployed in this study followed an approach that Tufts CSDD has been using to evaluate trends in, and the impact of, protocol design practice since 2008.
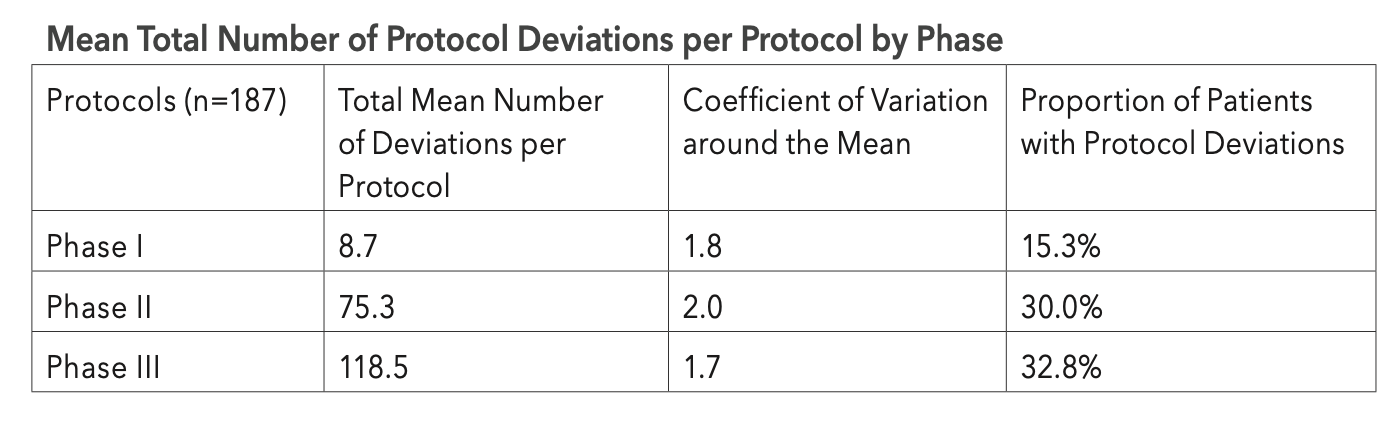
Data on a total of 187 protocols targeting a range of disease conditions was analyzed. One-quarter of the total were Phase I protocols, 39% Phase II, and 36% were Phase III protocols.
The table on the facing page provides high-level baseline measures of protocol deviation experience by clinical phase. Phase I protocols have the lowest mean number of deviations and involve half the proportion of patients than those observed in Phase II and Phase III protocols. Each Phase III protocol has a mean number of 119 total deviations, involving approximately one-third of all patients participating in that protocol. The coefficients of variation around the mean number are very high—between 1.7 and 2.0—indicating that the average deviations per protocol are widely dispersed and difficult to predict.
Wide variation in protocol deviation experience was also observed by therapeutic area. Phase II and III oncology protocols average almost 20% more total deviations than the average for non-oncology protocols, at 108.8 and 91.9, respectively. Protocol deviations in combined Phase II and III oncology protocols involve 47% of the total study volunteers, nearly double the proportion observed in non-oncology protocols. Rare disease indications average a lower relative number of total deviations (78.1) among a smaller proportion of study volunteers (27.7%) compared to that of non-rare disease indications.
Changing the Face—and Faces—of Clinical Research
The six steps to designing a successful diversity action plan for a clinical trial protocol.
How Often are Registered Clinical Trials Publishing Their Results?
Study examines how many registered clinical trials with published protocols are also publishing their results.

Shining a Light on the Inefficiencies in Amendment Implementation
Study findings elevate the need to optimize protocol amendment experience.

Navigating Toward a Digital Clinical Trial Protocol
While steady progress has been made in recent years, CROs and sponsors still have decisions to make on which solutions are best for them.

Master Protocols: Implementing Effective Treatment Adaptations in the Randomization
It is unrealistic to include infinite adaptations in an IRT system, thus identifying the optimal level of adaptations requires examination of the study’s characteristics and planning phase considerations.

Rethinking ECOG Scores to Improve Patient Access and Clinical Trials Eligibility
Recent study results highlight need for new metrics in ECOG scoring to mitigate variability.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

PF-06821497 Treatment Of Relapsed/Refractory SCLC, Castration Resistant Prostate Cancer, and Follicular Lymphoma
- Study Details
- Tabular View
- No Results Posted

Key Inclusion Criteria:
Histological or cytological diagnosis of advanced / metastatic solid tumor with the following tumor types in individual study parts:
Part 1A (closed to enrollment):
Part 1B (closed to enrollment):
- Castration resistant prostate cancer. Patients should have received either abiraterone and/or enzalutamide treatment and have evidence of prostate cancer progression (per PCWG3) Japan cohort
- Castration resistant prostate cancer that is resistant to SOC or for which no local regulatory approved SOC is available that would confer significant clinical benefit in the medical judgement of the investigator. Patients should have received either abiraterone and/or enzalutamide treatment and have evidence of prostate cancer progression (per PCWG3) China cohort
- Castration resistant prostate cancer that is intolerant/resistant to SOC or for which no local regulatory approved SOC is available that would confer significant clinical benefit in the medical judgement of the investigator. Patients who refused SOC may be eligible. Patients should have received either abiraterone and/or enzalutamide treatment and have evidence of prostate cancer progression (per PCWG3)
• Castration resistant prostate cancer. Patients should have received either abiraterone and/or enzalutamide treatment, may have received up to 1 line of chemotherapy and have evidence of prostate cancer progression (per PCWG3)
- Castration resistant prostate cancer. Patients should have received abiraterone treatment, may have received up to 1 prior line of chemotherapy, have not received prior enzalutamide, apalutamide or darolutamide and have evidence of prostate cancer progression (per PCWG3)
- Patients must have radiographic evidence of disease
Other inclusion criteria:
- Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 or 1.
- Adequate organ function
Key Exclusion Criteria:
- Prior Chemotherapy: Part 1C , Japan cohort and China cohort (CRPC): no more than 2 previous regimens of chemotherapy Part 2A: CRPC: no more than 1 previous regimen of systemic chemotherapy Part 2B (CRPC): no more than 1 previous regimen of chemotherapy
- Prior irradiation to >25% of the bone marrow.
- QTcF interval >480 msec at screening.
- Hypertension that cannot be controlled by medications (>150/90 mmHg despite optimal medical therapy).
- Known or suspected hypersensitivity to PF 06821497 or any components or enzalutamide (CRPC)
- Active inflammatory gastrointestinal disease, chronic diarrhea, known diverticular disease or previous gastric resection or lap band surgery. Gastroesophageal reflux disease under treatment with proton pump inhibitors is allowed.
- Current use or anticipated need for food or drugs that are known strong CYP3A4/5 inducers or inhibitors, including their administration within 10 days or 5 half lives of the CYP3A4/5 inhibitor, whichever is longer prior to first dose of investigational product.

- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
We’re on the road right now – join in on the fun and follow @thebrokebackpacker on IG!
- Meet the Team
- Work with Us
- Czech Republic
- Netherlands
- Switzerland
- Scandinavia
- Philippines
- South Korea
- New Zealand
- South Africa
- Budget Travel
- Work & Travel
- The Broke Backpacker Manifesto
- Travel Resources
- How to Travel on $10/day
Home » Europe » Moscow
EPIC MOSCOW Itinerary! (2024)
Moscow is the heart of Mother Russia. Just the mention of this city conjures images of colorful bulbous pointed domes, crisp temperatures, and a uniquely original spirit!
Moscow has an incredibly turbulent history, a seemingly resilient culture, and a unique enchantment that pulls countless tourists to the city each year! Although the warmer months make exploring Moscow’s attractions more favorable, there’s just something about a fresh snowfall that only enhances the appearance of the city’s iconic sites!
If you’re a first-time visitor to Moscow, or simply wanting to see as much of the city as possible, this Moscow itinerary will help you do just that!

Unlock Our GREATEST Travel Secrets!
Sign up for our newsletter and get the best travel tips delivered right to your inbox.
Best Time To Visit Moscow
Where to stay in moscow, moscow itinerary, day 1 itinerary in moscow, day 2 itinerary in moscow, day 3 and beyond, staying safe in moscow, day trips from moscow, faq on moscow itinerary.
Here is a quick look at the seasons so you can decide when to visit Moscow!
The summer months (June-August) are a great time to travel to Moscow to take advantage of the enjoyable mild temperatures. This is considered peak travel season. Bear in mind that hotel prices rise along with the temperatures!

If you’re planning a trip to Moscow during fall (September-November) try to plan for early fall. This way the temperatures will still be pleasant and winter won’t be threatening.
Russian winters (December-February) are not for the faint of heart as Napoleon learned to his peril. Some days the sun will be out for less than an hour, and snow is guaranteed. Although winters are exceptionally cold, this is when you’ll get a true glimpse of the Moscow experience!
The best time to visit Moscow is during spring (March-May). The temperatures will begin to creep up and the sun begins to shine for significant portions of the day. Hotel rates will also have yet to skyrocket into peak ranges!

With a Moscow City Pass , you can experience the best of Moscow at the CHEAPEST prices. Discounts, attractions, tickets, and even public transport are all standards in any good city pass – be sure invest now and save them $$$ when you arrive!
Moscow is a large city with many accommodation options to choose from. Staying in a location that fits with your travel plans will only enhance your Moscow itinerary. Here is a brief introduction to a few great areas of the city we recommend checking out!
The best place to stay in Moscow to be close to all the action is Kitay-Gorod. This charming neighborhood will put you within walking distance to Moscow’s famous Red Square, thus cutting down on travel time. This will allow you to see more of the city in a shorter amount of time!

It’s surrounded by restaurants, cafes, bars, and shops. If you’re a first-time visitor to Moscow, or just planning a quick weekend in Moscow, then this area is perfect for you!
Another great area to consider is the Zamoskvorechye district. This area of the city offers a blend of new and old Moscow. It has an artsy vibe and there are plenty of fun sites you can explore outside of the main touristy areas of Moscow.
Of course, as in all areas of Moscow, it’s close to public transportation that will quickly connect you with the rest of the city and make your Moscow itinerary super accessible!
Best Airbnb in Moscow – Exclusive Apartment in Old Moscow

Modern and cozy, this apartment is in the heart of Old Moscow. Bordering the Basmanny and Kitay-Gorod districts, this two-bedroom flat is walking distance to the Kremlin and Red Square. Safe, quiet, and comfortable, this is the best Airbnb in Moscow, no question!
Best Budget Hotel in Moscow – Izmailovo Alfa Hotel

The Izmailovo Alfa Hotel is a very highly rated accommodation that provides all the components necessary for a comfortable trip to Moscow. There is an on-site restaurant, bar, fitness center, and an airport shuttle service. The rooms are modern and spacious and are equipped with a TV, heating/air conditioning, minibar, and more!
Best Luxury Hotel in Moscow – Crowne Plaza Moscow World Trade Centre

If you’re touring Moscow in luxury, the Crowne Plaza Moscow World Trade Centre is the hotel for you! Elegantly furnished rooms are equipped with a minibar, flat-screen TV, in-room safes, as well as tea and coffee making facilities! Bathrooms come with bathrobes, slippers, and free toiletries. There is also an onsite restaurant, bar, and fitness center.
Best Hostel in Moscow – Godzillas Hostel

Godzillas Hostel is located in the center of Moscow, just a short walk from all the major tourist attractions and the metro station. Guests will enjoy all the usual hostel perks such as self-catering facilities, 24-hour reception, Free Wi-Fi, and security lockers. This is one of the best hostels in Moscow and its wonderful social atmosphere and will make your vacation in Moscow extra special!
Godzillas Hostel is one of our favourites in Moscow but they’re not taking guests right now. We’re not sure if they’re closed for good but we hope they’ll come back soon.
An important aspect of planning any trip is figuring out the transportation situation. You’re probably wondering how you’re going to get to all of your Moscow points of interest right? Luckily, this sprawling city has an excellent network of public transportation that will make traveling a breeze!
The underground metro system is the quickest and most efficient way to travel around Moscow. Most visitors rely exclusively on this super-efficient transportation system, which allows you to get to pretty much anywhere in the city! It’s also a great option if you’re planning a Moscow itinerary during the colder months, as you’ll be sheltered from the snow and freezing temperatures!

If you prefer above-ground transportation, buses, trams, and trolleybuses, run throughout the city and provide a rather comfortable alternative to the metro.
Moscow’s metro, buses, trams, and trolleybuses are all accessible with a ‘Troika’ card. This card can be topped up with any sum of money at a metro cash desk. The ticket is simple, convenient, and even refundable upon return to a cashier!
No matter which method you choose, you’ll never find yourself without an easy means of getting from point A to point B!
Red Square | Moscow Kremlin | Lenin’s Mausoleum | St. Basil’s Cathedral | GUM Department Store
Spend the first day of your itinerary taking your own self guided Moscow walking tour around the historic Red Square! This is Moscow’s compact city center and every stop on this list is within easy walking distance to the next! Get ready to see all of the top Moscow landmarks!
Day 1 / Stop 1 – The Red Square
- Why it’s awesome: The Red Square is the most recognizable area in Moscow, it has mesmerizing architecture and centuries worth of history attached to its name.
- Cost: Free to walk around, individual attractions in the square have separate fees.
- Food nearby: Check out Bar BQ Cafe for friendly service and good food in a great location! The atmosphere is upbeat and they’re open 24/7!
The Red Square is Moscow’s historic fortress and the center of the Russian government. The origins of the square date back to the late 15th century, when Ivan the Great decided to expand the Kremlin to reflect Moscow’s growing power and prestige!
During the 20th century, the square became famous as the site for demonstrations designed to showcase Soviet strength. Visiting the Red Square today, you’ll find it teeming with tourists, who come to witness its magical architecture up close!

The square is the picture postcard of Russian tourism, so make sure to bring your camera when you visit! No matter the season, or the time of day, it’s delightfully photogenic!
It’s also home to some of Russia’s most distinguishing and important landmarks, which we’ve made sure to include further down in this itinerary. It’s an important center of Russia’s cultural life and one of the top places to visit in Moscow!
In 1990, UNESCO designated Russia’s Red Square as a World Heritage site. Visiting this historic site is a true bucket-list event and essential addition to your itinerary for Moscow!
Day 1 / Stop 2 – The Moscow Kremlin
- Why it’s awesome: The Moscow Kremlin complex includes several palaces and cathedrals and is surrounded by the Kremlin wall. It also houses the principal museum of Russia (the Kremlin Armory).
- Cost: USD $15.00
- Food nearby: Bosco Cafe is a charming place to grat a casual bite to eat. They have excellent coffee and wonderful views of the Red Square and the Moscow Kremlin!
The iconic Moscow Kremlin , also known as the Kremlin museum complex, sits on Borovitsky Hill, rising above the Moscow River. It is a fortified complex in the center of the city, overlooking several iconic buildings in the Red Square!
It’s the best known of the Russian Kremlins – citadels or fortress’ protecting and dominating a city. During the early decades of the Soviet era, the Kremlin was a private enclave where the state’s governing elite lived and worked.
The Kremlin is outlined by an irregularly shaped triangular wall that encloses an area of 68 acres! The existing walls and towers were built from 1485 to 1495. Inside the Kremlin museum complex, there are five palaces, four cathedrals, and the enclosing Kremlin Wall with Kremlin towers.
The Armoury Chamber is a part of the Grand Kremlin Palace’s complex and is one of the oldest museums of Moscow, established in 1851. It showcases Russian history and displays many cherished relics. Definitely make sure to check out this museum while you’re here!

The churches inside the Moscow Kremlin are the Cathedral of the Dormition, Church of the Archangel, Church of the Annunciation, and the bell tower of Ivan Veliki (a church tower).
The five-domed Cathedral of the Dormition is considered the most famous. It was built from 1475–1479 by an Italian architect and has served as a wedding and coronation place for great princes, tsars, and emperors of Russia. Church services are given in the Kremlin’s numerous cathedrals on a regular basis.
The Grand Kremlin Palace was the former Tsar’s Moscow residence and today it serves as the official workplace of the President of the Russian Federation (Vladimir Putin seems to have bagged that title for life) .
Insider Tip: The Kremlin is closed every Thursday! Make sure to plan this stop on your Moscow itinerary for any other day of the week!
Day 1 / Stop 3 – Lenin’s Mausoleum
- Why it’s awesome: The mausoleum displays the preserved body of Soviet leader Vladimir Lenin .
- Cost: Free!
- Food nearby: Khinkal’naya is a charming Georgian restaurant with vaulted ceilings and exposed brick. It’s a popular place with locals and right next to the Red Square!
Lenin’s Mausoleum, also known as Lenin’s Tomb, is the modernist mausoleum for the revolutionary leader Vladimir Lenin. It’s located within the Red Square and serves as the resting place for the Soviet leader! His preserved body has been on public display since shortly after his death in 1924.
It’s located just a few steps away from the Kremlin Wall and is one of the most controversial yet popular Moscow attractions!
Admission is free for everyone, you’ll only need to pay if you need to check a bag. Before visitors are allowed to enter the mausoleum, they have to go through a metal detector first. No metal objects, liquids, or large bags are allowed in the mausoleum!

Expect a line to enter the building, and while you’re inside the building, you’ll be constantly moving in line with other visitors. This means you won’t be able to spend as long as you’d like viewing the mausoleum, but you’ll still be able to get a good look. Pictures and filming while inside the building are strictly prohibited, and security guards will stop you if they see you breaking this rule.
The mausoleum is only open on Tuesday, Wednesday, Thursday, and Saturday – unless it’s a public holiday or a day scheduled for maintenance. The hours it’s open for each day are limited, make sure to check online before you visit to make sure you can fit this into your Moscow itinerary for that day!
Insider Tip: The Lenin’s Museum is there for people to pay their respect; remember to keep silent and move along quickly, it’s not intended for people to congregate around. Also, men are not allowed to wear hats and everyone must take their hands out of their pockets when inside the building.
Day 1 / Stop 4 – St. Basil’s Cathedral
- Why it’s awesome: A dazzling designed cathedral that showcases Russia’s unique architecture. This cathedral is one of the most recognizable symbols of the country!
- Cost: USD $8.00
- Food nearby: Moskovskiy Chaynyy Klub is a cozy cafe serving food items and pipping hot tea; it’s the perfect place to go if you’re visiting Moscow during the winter months!
Located in the Red Square, the ornate 16th-century St. Basil’s Cathedral is probably the building you picture when you think of Moscow’s unique architecture. Its colorful onion-shaped domes tower over the Moscow skyline!
The cathedral was built from 1555-1561 by order of Tsar Ivan the Terrible. It was designed with an iconic onion dome facade and enchanting colors that captivate all who see it. Fun fact: If you’re wondering why Russian churches have onion domes, they are popularly believed to symbolize burning candles!
This iconic cathedral has become a symbol of Russia due to its distinguishing architecture and prominent position inside the Red Square. It’s one of the most beautiful, wonderful, and mesmerizing historical cathedrals in the world!

The interior of the church surprises most people when they visit. In contrast to the large exterior, the inside is not so much one large area, but rather a collection of smaller areas, with many corridors and small rooms. There are 9 small chapels and one mausoleum grouped around a central tower.
Visiting the inside is like walking through a maze, there are even small signs all around the cathedral tracing where to walk, and pointing you in the right direction! The walls are meticulously decorated and painted with intricate floral designs and religious themes.
The church rarely holds service and is instead a museum open for the public to visit.
Insider Tip: During the summer months the line to go inside the cathedral can get quite long! Make sure to arrive early or reserve your tickets online to guarantee quick access into the cathedral!
Day 1 / Stop 5 – GUM Department Store
- Why it’s awesome: This is Russia’s most famous shopping mall! It’s designed with elegant and opulent architecture and provides a real sense of nostalgia!
- Cost: Free to enter
- Food nearby: Stolovaya 57 is a cafeteria-style restaurant with a variety of inexpensive Russian cuisine menu items including soups, salads, meat dishes, and desserts. It’s also located inside the GUM department store, making it very easily accessible when you’re shopping!
The enormous GUM Department Store is located within the historic Red Square. It has a whimsical enchantment to it that sets it apart from your typical department store.
A massive domed glass ceiling lines the top of the building and fills the interior with natural sunlight. There are live plants and flowers placed throughout the mall that give the shopping complex a lively and cheerful feel! A playful fountain sits in the center, further adding to the malls inviting a sense of wonder and amusement!
The GUM department store opened on December 2, 1893. Today, it includes local and luxury stores, including Fendi, Louis Vuitton, Prada, and many more! There are numerous cafes, restaurants, and even a movie theater inside!

For a special treat, head into Gastronom 1. This 1950s-style shop sells gourmet food items, like wine, freshly-baked pastries, cheese, Russian chocolate, and of course, vodka! Also, be on the lookout for a bicycle pedaling ice cream truck with an employing selling ice cream!
The ambiance is simply amazing, a trip to this idyllic shopping mall is an absolute must on any Moscow itinerary!
Insider Tip: Make sure to carry some small change on you in case you need to use the restroom, you’ll need to pay 50 rubles – or about USD $0.80 to use the bathroom in GUM.

Wanna know how to pack like a pro? Well for a start you need the right gear….
These are packing cubes for the globetrotters and compression sacks for the real adventurers – these babies are a traveller’s best kept secret. They organise yo’ packing and minimise volume too so you can pack MORE.
Or, y’know… you can stick to just chucking it all in your backpack…
Novodevichy Convent | Gorky Park | State Tretyakov Gallery | All-Russian Exhibition Center | Bolshoi Theater
On your 2 day itinerary in Moscow, you’ll have a chance to use the city’s excellent public transportation service! You’ll explore a few more of Moscow’s historic highlight as well as some modern attractions. These sites are a little more spread out, but still very easily accessible thanks to the metro!
Day 2 / Stop 1 – Novodevichy Convent
- Why it’s awesome: The Novodevichy Convent is rich in imperial Russian history and contains some of Russia’s best examples of classical architecture!
- Cost: USD $5.00
- Food nearby: Culinary Shop Karavaevs Brothers is a cozy and simple place to have a quick bite, they also have vegetarian options!
The Novodevichy Convent is the best-known and most popular cloister of Moscow. The convent complex is contained within high walls, and there are many attractions this site is known for!
The six-pillared five-domed Smolensk Cathedral is the main attraction. It was built to resemble the Kremlin’s Assumption Cathedral and its facade boasts beautiful snowy white walls and a pristine golden onion dome as its centerpiece. It’s the oldest structure in the convent, built from 1524 -1525, and is situated in the center of the complex between the two entrance gates.
There are other churches inside the convent as well, all dating back from many centuries past. The convent is filled with an abundance of 16th and 17th-century religious artworks, including numerous large and extravagant frescos!

Just outside the convent’s grounds lies the Novodevichy Cemetery. Here, you can visit the graves of famous Russians, including esteemed authors, composers, and politicians. Probably the most intriguing gravestone belongs to Russian politician Nikita Khruschev!
The Novodevichy Convent is located near the Moscow River and offers a peaceful retreat from the busy city. In 2004, it was proclaimed a UNESCO World Heritage Site. The convent remains remarkably well-preserved and is an outstanding example of Moscow Baroque architecture!
Insider Tip: To enter the cathedrals inside the complex, women are advised to cover their heads and shoulders, while men should wear long pants.
Day 2 / Stop 2 – Gorky Central Park of Culture and Leisure
- Why it’s awesome: A large amusement area in the heart of the city offering many attractions!
- Cost: Free!
- Food nearby: Check out Mepkato, located inside Gorky Central Park for a casual meal in a cozy setting. There are indoor and outdoor seating options and the restaurant is child-friendly!
Gorky Central Park of Culture and Leisure is a large green space in the heart of Moscow. The park opened in 1928, and it stretches along the scenic embankment of the Moskva River. It covers an area of 300-acres and offers a lovely contrast from the compact city center.
You’ll find all sorts of wonderful attractions, from boat rides to bike rentals to tennis courts and ping-pong tables, and much more! there are an open-air cinema and festive events and concerts scheduled in the summer months. A wide selection of free fitness classes is also offered on a regular basis, including jogging, roller skating, and dancing!
Although many of the options you’ll find here are more suited for outdoor leisure during the summer, you’ll also a selection of winter attractions, including one of Europe’s largest ice rinks for ice-skating!

If you’re trying to decide what to do in Moscow with kids, the park also offers several venues designed specifically for kids. Check out the year-round Green School which offers hands-on classes in gardening and art! You can also feed the squirrels and birds at the Golitsinsky Ponds!
The park is very well maintained and kept clean and the entrance is free of charge, although most individual attractions cost money. There is also Wi-Fi available throughout the park.
With so many attractions, you could easily spend all day here! If you’re only planning a 2 day itinerary in Moscow, make sure to plan your time accordingly and map out all the areas you want to see beforehand!
Day 2 / Stop 3 – The State Tretyakov Gallery
- Why it’s awesome: The gallery’s collection consists entirely of Russian art made by Russian artists!
- Food nearby : Brothers Tretyakovs is located right across the street from the gallery. It’s a wonderfully atmospheric restaurant serving top quality food and drinks!
The State Tretyakov Gallery was founded in 1856 by influential merchant and collector Pavel Tretyakov. The gallery is a national treasury of Russian fine art and one of the most important museums in Russia!
It houses the world’s best collection of Russian art and contains more than 130, 000 paintings, sculptures, and graphics! These works have been created throughout the centuries by generations of Russia’s most talented artists!

The exhibits range from mysterious 12th-century images to politically charged canvases. The collection is rich and revealing and offers great insight into the history and attitudes of this long-suffering yet inspired people!
All pictures are also labeled in English. If you plan to take your time and see everything inside the museum it will take a good 3-4 hours, so make sure to plan your Moscow trip itinerary accordingly! This gallery is a must-see stop for art lovers, or anyone wanting to explore the local culture and history of Russia in a creative and insightful manner!
Insider Tip: When planning your 2 days in Moscow itinerary, keep in mind that most museums in Moscow are closed on Mondays, this includes The State Tretyakov Gallery!
Day 2 / Stop 4 – All-Russian Exhibition Center
- Why it’s awesome: This large exhibition center showcases the achievements of the Soviet Union in several different spheres.
- Food nearby: Varenichnaya No. 1 serves authentic and homestyle Russian cuisine in an intimate and casual setting.
The All-Russian Exhibition Center is a massive park that presents the glory of the Soviet era! It pays homage to the achievements of Soviet Russia with its many different sites found on the property.
The center was officially opened in 1939 to exhibit the achievements of the Soviet Union. It’s a huge complex of buildings and the largest exhibition center in Moscow. There are several exhibition halls dedicated to different achievements and every year there are more than one hundred and fifty specialized exhibitions!

The Peoples Friendship Fountain was constructed in 1954 and is a highlight of the park. The stunning gold fountain features 16 gilded statues of girls, each representing the former Soviet Union republics.
The Stone Flower Fountain was also built in 1954 and is worth checking out. The centerpiece of this large fountain is a flower carved from stones from the Ural Mountains! Along the side of the fountain are various bronze sculptures.
You will find many people zipping around on rollerblades and bicycles across the large area that the venue covers. It’s also home to amusement rides and carousels, making it the perfect place to stop with kids on your Moscow itinerary! Make sure to wear comfortable shoes and allow a few hours to explore all the areas that interest you!
Day 2 / Stop 5 – Bolshoi Theater
- Why it’s awesome: The Bolshoi Theater is a historic venue that hosts world-class ballet and opera performances!
- Cost: Prices vary largely between USD $2.00 – USD $228.00 based on seat location.
- Food nearby: Head to the Russian restaurant, Bolshoi for high-quality food and drinks and excellent service!
The Bolshoi Theater is among the oldest and most renowned ballet and opera companies in the world! It also boasts the world’s biggest ballet company, with more than 200 dancers!
The theater has been rebuilt and renovated several times during its long history. In 2011 it finished its most recent renovation after an extensive six-year restoration that started in 2005. The renovation included an improvement in acoustics and the restoration of the original Imperial decor.
The Bolshoi Theater has put on many of the world’s most famous ballet acts! Tchaikovsky’s ballet Swan Lake premiered at the theater in 1877 and other notable performances of the Bolshoi repertoire include Tchaikovsky’s The Sleeping Beauty and The Nutcracker!

Today, when you visit the theater, you can expect a magical performance from skilled singers, dancers, and musicians with the highest level of technique!
If you don’t have time to see a show, the theater also provides guided tours on select days of the week. Tours are given in both Russian and English and will provide visitors with a more intimate look at the different areas of the theater!
The stage of this iconic Russian theater has seen many outstanding performances. If you’re a fan of the performing arts, the Bolshoi Theater is one of the greatest and oldest ballet and opera companies in the world, making it a must-see attraction on your Moscow itinerary!

Godzillas Hostel
Godzillas Hostel is located in the center of Moscow, just a short walk from all the major tourist attractions and the metro station.
- Towels Included
Cosmonautics Museum | Alexander Garden | Ostankino Tower | Izmaylovo District | Soviet Arcade Museum
Now that we’ve covered what to do in Moscow in 2 days, if you’re able to spend more time in the city you’re going to need more attractions to fill your time. Here are a few more really cool things to do in Moscow we recommend!
Memorial Museum of Cosmonautics
- Hear the timeline of the ‘space race’ from the Russian perspective
- This museum is fun for both adults and children!
- Admission is USD $4.00
The Memorial Museum of Cosmonautics is a museum dedicated to space exploration! The museum explores the history of flight, astronomy, space exploration, space technology, and space in the arts. It houses a large assortment of Soviet and Russian space-related exhibits, and the museum’s collection holds approximately 85,000 different items!

The museum does an excellent job of telling the full story of the exciting space race between the USSR and the US! It highlights the brightest moments in Russian history and humanity and is very interesting and fun for all ages!
If you’re a fan of space or just curious about gaining insight into Russia’s fascinating history of space exploration, make sure to add this to your 3 day itinerary in Moscow!
The Alexander Garden
- A tranquil place to relax near the Red Square
- Green lawns dotted with sculptures and lovely water features
- The park is open every day and has no entrance fee
The Alexander Garden was one of the first urban public parks in Moscow! The garden premiered in 1821 and was built to celebrate Russia’s victory over Napoleon’s forces in 1812!
The park is beautiful and well maintained with paths to walk on and benches to rest on. The park contains three separate gardens: the upper garden, middle garden, and lower garden.

Located in the upper garden, towards the main entrance to the park is the Tomb of the Unknown Soldier with its eternal flame. This monument was created in 1967 and contains the body of a soldier who fell during the Great Patriotic War!
The park stretches along all the length of the western Kremlin wall for about half a mile. Due to its central location in the city, it’ll be easily accessible when you’re out exploring The Red Square.
It provides a bit of relief from the city’s high-energy city streets. Bring a picnic lunch, go for a walk, or just sit and people watch, this is one of the best Moscow sites to wind-down and relax!
Ostankino Television Tower
- Television and radio tower in Moscow
- Currently the tallest free-standing structure in Europe
- Make sure you bring your passport when you visit, you can’t go up without it!
For spectacular views of the city, make sure to add the Ostankino Television Tower to your itinerary for Moscow! This impressive free-standing structure provides stunning views of the city in every direction. The glass floor at the top also provides great alternative views of the city!

It takes just 58 seconds for visitors to reach the Tower’s observation deck by super fast elevator. The tower is open every day for long hours and is a great site in Moscow to check out! There is even a restaurant at the top where you can enjoy rotating views of the city while you dine on traditional Russian cuisine or European cuisine!
The tower is somewhat of an architectural surprise in a city that is not known for skyscrapers! To see the city from a new perspective, make sure to add this stop to your Moscow itinerary!
Izmaylovo District
- The most popular attractions in this district are the kremlin and the flea market
- Outside of the city center and easy to reach via metro
- Most popular during the summer and on weekends
Travel outside the city center and discover a unique area of the city! The Izmaylovo District is a popular destination for locals and tourists alike, and one of the coolest places to see in Moscow! The two main attractions we recommend checking out are the Kremlin and the flea market.
The Izmailovo Kremlin was established as a cultural center and molded after traditional Russian architecture. This colorful complex is home to several single-subject museums, including a Russian folk art museum and a vodka museum!

Next to the Kremlin is the Izmailovo open-air market, which dates back to the 17th century! The market is connected to the Izmailovo Kremlin by a wooden bridge. Pick up all your Russian souvenirs here, including traditional handicrafts, paintings, books, retro toys, and Soviet memorabilia!
You will find many hand-made and hand-painted options available at higher prices, as well as mass-produced souvenir options at lower prices!
Museum of Soviet Arcade Games
- Closed on Mondays
- Filled with old arcade games that visitors get to try out!
- The museum also includes a small cafe and burger shop
For something a little different, check out the Museum of Soviet Arcade Games! The museum features roughly 60 machines from the Soviet era, including video games, pinball machines, and collaborative hockey foosball! The machines inside the museum were produced in the USSR in the mid-1970s.

The best part is, most of the games are still playable! Purchase tickets and try the games out for yourself! The museum also has a neat little screening room that plays old Soviet cartoons and an area with Soviet magazines! This unique attraction is a fun addition to a 3 day itinerary in Moscow, and an attraction that all ages will enjoy!
Whether you’re spending one day in Moscow, or more, safety is an important thing to keep in mind when traveling to a big city! Overall, Moscow is a very safe place to visit. However, it is always recommended that tourists take certain precautions when traveling to a new destination!
The police in Moscow is extremely effective at making the city a safe place to visit and do their best to patrol all of the top Moscow, Russia tourist attractions. However, tourists can still be a target for pickpockets and scammers.
Moscow has a huge flow of tourists, therefore there is a risk for pickpocketing. Simple precautions will help eliminate your chances of being robbed. Stay vigilant, keep your items close to you at all times, and don’t flash your valuables!
If you’re planning a solo Moscow itinerary, you should have no need to worry, as the city is also considered safe for solo travelers, even women. Stay in the populated areas, try and not travel alone late at night, and never accept rides from strangers or taxis without a meter and correct signage.
The threat of natural disasters in Moscow is low, with the exception of severe winters when the temperature can dip below freezing! Bring a good, warm jacket if you visit in Winter.
However, please note that Russian views on homsexuality are far less accepting than those in Western Europe. Likewise, Non-Caucasian travellers may sadly encounter racism in Russia .
Don’t Forget Your Travel Insurance for Moscow
ALWAYS sort out your backpacker insurance before your trip. There’s plenty to choose from in that department, but a good place to start is Safety Wing .
They offer month-to-month payments, no lock-in contracts, and require absolutely no itineraries: that’s the exact kind of insurance long-term travellers and digital nomads need.

SafetyWing is cheap, easy, and admin-free: just sign up lickety-split so you can get back to it!
Click the button below to learn more about SafetyWing’s setup or read our insider review for the full tasty scoop.
Now that we’ve covered all the top things to see in Moscow, we thought we’d include some exciting day trips to other areas of the country!
Sergiev Posad (Golden Ring)

On this 7-hour guided tour, you’ll visit several scenic and historic areas of Russia. Start your day with hotel pick-up as you’re transferred by a comfortable car or minivan to Sergiev Posad. Admire the charming Russian countryside on your drive and enjoy a quick stop to visit the Russian village, Rudonezh!
You’ll see the majestic Saint Spring and the Church of Sergiev Radonezh. You’ll also visit the UNESCO World Heritage Site, Trinity Lavra of St. Sergius, one of the most famous Orthodox sites in Russia!
Lastly, you’ll swing by the local Matreshka market and enjoy a break in a nice Russian restaurant before returning to Moscow!
Day Trip to Vladimir and Suzdal

On this 13-hour trip, you’ll discover old Russia, with its picturesque landscapes and white-stoned beautiful churches! You’ll visit the main towns of the famous Golden Ring of Russia – the name for several cities and smaller towns north-east of Moscow.
Your first stop will be in the town of Vladimir, the ancient capital of all Russian principalities. The city dates back to the 11th century and is one of the oldest and the most important towns along the Ring! Next, you’ll visit Suzdal, a calm ancient Russian town north of Vladimir with only 13,000 inhabitants!
The old-style architecture and buildings of Suzdal are kept wonderfully intact. If you’re spending three days in Moscow, or more, this is a great option for exploring the charming areas outside the city!
Zvenigorod Day Trip and Russian Countryside

On this 9-hour private tour, you’ll explore the ancient town of Zvenigorod, one of the oldest towns in the Moscow region! As you leave Moscow you’ll enjoy the stunning scenery along the Moscow River, and make a few stops at old churches along the way to Zvenigorod.
Upon arrival, you’ll explore the medieval center, including the 14th-century Savvino-Storozhevsky Monastery. Next, you’ll take a break for lunch (own expense) where you’ll have the chance to try out the Russian cuisine! Next, you’ll visit the Museum of Russian Dessert and sip on tea at a Russian tea ceremony.
The final stop of the day is at the Ershovo Estate, a gorgeous place to walk around and enjoy nature!
Day Trip to St Petersburg by Train visiting Hermitage & Faberge

On this full-day tour, you’ll enjoy a a full round trip to St Petersburg where you’ll spend an exciting day exploring another popular Russian city! You’ll be picked up from your hotel in Moscow and be transferred to the train station where you’ll ride the high-speed train ‘Sapsan’ to St Petersburg.
Upon arrival, you’ll start the day by touring the Hermitage Museum and the Winter Palace. Next, you’ll visit the Faberge Museum, where you’ll explore the impressive collection of rare Faberge Eggs! In the afternoon, enjoy a sightseeing boat ride and a traditional 3-course Russian lunch.
If you’re spending 3 days in Moscow, or more, this is an excellent trip to take!
Trip to Kolomna – Authentic Cultural Experience from Moscow

On this 10-hour tour, you’ll escape the city and travel to the historic town of Kolomna! First, you’ll visit the 14th-century Kolomna Kremlin, home to the Assumption Cathedral and an abundance of museums!
Next, enjoy lunch at a local cafe (own expense) before embarking on a tour of the Marshmallow Museum – of course, a marshmallow tasting is provided! Your final stop is the Museum of Forging Settlements, where displays include armor and accessories for fishing and hunting.
Discover this beautiful Russian fairytale city on a private trip, where all of the planning is taken care of for you!

Stash your cash safely with this money belt. It will keep your valuables safely concealed, no matter where you go.
It looks exactly like a normal belt except for a SECRET interior pocket perfectly designed to hide a wad of cash, a passport photocopy or anything else you may wish to hide. Never get caught with your pants down again! (Unless you want to…)
Find out what people want to know when planning their Moscow itinerary.
How many days you need in Moscow?
We recommend that you spend at least two or three days in Moscow to take it all in.
What’s the best month to visit Moscow?
The best time to visit Moscow is over the spring, from March to May as temperatures are mild, crowds are thin and prices are reasonable.
What are some unusual things to do in Moscow?
I mean, queuing up to see an almost 100 year old corpse is pretty unsual! Check out Lenin’s Mausoleum if you fancy it!
What are some fun things to do in Moscow?
The Memorial Museum of Cosmonautics is a fun place to explore the famous space race from the perspective of the ‘other side’!
We hope you enjoyed our Moscow itinerary! We’ve made sure to cover all the Moscow must-sees as well as some unique attractions in the city! Our addition of insider tips, favorite food stops, and day trips from Moscow is an added bonus and will guarantee you make the most out of your exciting Russian vacation!
Immerse yourself in the modern and traditional Russian lifestyle! Get lost in museums, witness awe-inspiring architecture, and indulge in Russian cuisine! Spend the day strolling through all of the charming sites of Moscow, admiring the beautiful scenery and discovering the city’s fairytale-like enchantment!

And for transparency’s sake, please know that some of the links in our content are affiliate links . That means that if you book your accommodation, buy your gear, or sort your insurance through our link, we earn a small commission (at no extra cost to you). That said, we only link to the gear we trust and never recommend services we don’t believe are up to scratch. Again, thank you!

Alya and Campbell

Share or save this post

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of followup comments via e-mail.
- Find-a-Dentist
Your Baby's First Dental Visit
Your baby is hitting new milestones every day, and his or her first dental visit is another one to include in the baby book!
Your child’s first dental visit should take place after that first tooth appears , but no later than the first birthday. Why so early? As soon as your baby has teeth , he or she can get cavities. Being proactive about your child’s dental health today can help keep his or her smile healthy for life. (Need a dentist? Use our Find-A-Dentist tool to find one in your area.)
How to Prepare
Moms and dads can prepare, too. When making the appointment, it can’t hurt to ask for any necessary patient forms ahead of time. It may be quicker and easier for you to fill them out at home instead of at the office on the day of your visit.
Make a list of questions, as well. If your child is teething , sucking his or her thumb or using a pacifier too much, your dentist can offer some advice.
What to Expect During the Visit
If your child cries a little or wiggles during the exam, don’t worry. It’s normal, and your dental team understands this is a new experience for your child!
Tips for a Great Visit
- Don’t schedule an appointment during naptime. Instead, pick a time your child is usually well-rested and cooperative.
- Make sure your child has had a light meal and brushes their teeth before their appointment so they won’t be hungry during their visit.
- Save snacks for after the visit so they aren’t on your child’s teeth during the exam.
- Think of the appointment as a happy and fun experience. If your child becomes upset during the visit, work with your dentist to calm your child. You’re on the same team!

The Clinton-Yeltsin Moscow Summit, January 1994

Declassified transcripts show close cooperation on nuclear and regional security issues, rising concerns about failure of economic reform in Russia
Top Clinton advisor called Yeltsin “arguably your most important foreign counterpart”
Washington, D.C., January 25, 2024 - Declassified highest-level records from the Moscow summit 30 years ago this month detail U.S. President Bill Clinton’s strong personal support for Russian President Boris Yeltsin, their close cooperation on security issues, and deep concern about Yeltsin backtracking on economic reforms newly understood by the Clinton team as too “harsh” on the Russian people.
The documents include verbatim transcripts of Clinton’s two “one-on-one” discussions with Yeltsin, their trilateral discussion with Ukrainian President Leonid Kravchuk about removal of nuclear weapons from Ukraine, the detailed report from the U.S. Embassy Moscow on the dinner thrown by Yeltsin at his official dacha for Clinton, and the transcript of the expanded bilateral discussion between Clinton and Yeltsin on security issues.
The e-book includes an overview briefing memo for the President from national security adviser Anthony Lake, which describes Yeltsin as “arguably your most important foreign counterpart,” and the economic briefing memo to Clinton that admits that market reforms urged by the U.S. and implemented by Yeltsin failed to provide a social safety net for Russians, who reacted by voting against the reformers in the December 1993 parliamentary elections.
One highlight among the documents from January 1994 is the 12-page “eyes only” memo from Strobe Talbott to Secretary of State Warren Christopher, with Christopher’s extensive handwritten comments in the margins, including the admission that “set speeches” were “a real weakness” of his. Just a few days after being nominated to be Christopher’s deputy, a major promotion after less than one year as ambassador for the former Soviet republics, Talbott provides his boss with an almost anthropological account of Washington’s foreign policy village, with candid commentary on Russia and NATO policies (and their critics), on State Department personnel issues, and on internal tensions in the Clinton team. These included Lake’s “runs” at “knocking me out of Presidential events on Russia,” such as the upcoming Moscow one-on-ones. [1]
The new documents come from two major sources: a successful National Security Archive lawsuit against the State Department under the Freedom of Information Act and multiple declassification review requests filed at the Clinton Presidential Library. These records are highlights from the forthcoming 2,500-document declassified reference collection: U.S.-Russian Relations from the End of the Soviet Union to the Rise of Vladimir Putin , the next installment in the award-winning Digital National Security Archive series published by ProQuest.
The documents show the American team working hard to include multiple non-Yeltsin-centered events in the summit schedule. The U.S. ambassador, Thomas Pickering, hosted a reception at Spaso House for Clinton to meet oppositionists, excluding only Vladimir Zhirinovsky, leader of the extremist Liberal Democratic Party of Russia, the top vote-getter in the December legislative election. Clinton also addressed an audience of young Russians at the Ostankino television complex and met with the Patriarch of the Russian Orthodox Church, who had attempted to mediate the constitutional crisis between Yeltsin and the Supreme Soviet the previous year. [2]
Two of the documents, the Clinton-Kravchuk memcon at Kyiv’s Borispol Airport and the trilateral memcon with Clinton, Yeltsin and Kravchuk in Moscow, mark a key moment in the history of nuclear weapons in Ukraine. Traumatized by the 1986 Chernobyl explosion, the Ukrainian independence movement had pushed to remove Soviet nuclear weapons from Ukraine, and the newly independent state signed the Lisbon Protocol in May 1992 to become a non-nuclear party to the Non-Proliferation Treaty (along with Belarus and Kazakhstan, which also inherited Soviet nukes). Ukraine had no capacity to service and maintain the nuclear warheads—which were reaching the end of their service lives and were thus mini-Chernobyls waiting to happen—and couldn’t afford to build a nuclear reprocessing cycle (the Ukrainian Academy of Sciences’ estimate was $3 billion), especially with the international sanctions that would have ensued. [3]
In order to remove the nukes, Ukraine needed compensation and security assurances; at the same time, some voices in the Ukrainian parliament, the Verkhovna Rada, argued for keeping the nukes. The Moscow summit documents, including the Trilateral Statement signed by the three leaders, show the first steps towards the ultimate deal. The U.S. put up $60 million to prime the pump; the Russians provided fuel assemblies blended down from warhead fissile material to fuel Ukrainian nuclear power plants; and the Ukrainians started shipping warheads to the Russians for reprocessing. Ukraine also received debt forgiveness for hundreds of millions of dollars in already supplied Russian oil and gas and security assurances that lasted until 2014 when Russia annexed Crimea. The Russian invasion of Ukraine, in 2022, popularized the notion that Ukraine should have kept its nukes, but the record shows that maintaining a nuclear arsenal wasn’t really an option for the country in 1994. [4]
The biggest worry among the Clinton team at the Moscow summit was not so much the Ukraine trilateral but the fate of economic and democratic reforms in Russia after the shock of the December elections. During the opening dinner at Yeltsin’s dacha on January 13, the Russian president referred to the leading reformer, former prime minister Yegor Gaidar, as the leader of the government party in the Duma, “clearly impl[ying] that Gaidar would be out of the government and work only in the Duma.” The next day, during the formal Kremlin dinner, Clinton’s aides heard from Gaidar that, actually, he was being fired, and others of his team were also on their way out. At the insistence of Treasury undersecretary Larry Summers, Clinton sought a final one-on-one with Yeltsin on January 15 to warn that “President Clinton’s credibility was connected to President Yeltsin’s indication that he would continue the reforms, which were linked to a specific team of people.” But, of course, that was for Yeltsin to decide. [5]
The Documents

Clinton Presidential Library
This cover memo from the national security adviser for Clinton’s briefing book on the Moscow Summit highlights the major differences from the two previous Clinton-Yeltsin meetings at Vancouver and Tokyo in 1993. The challenge “at this critical turning point,” according to Lake, will be to reaffirm “a close U.S.-Russia partnership built on a Russian commitment to democratic political and market reform.” The parliamentary elections in December—a shocking loss for the reformers—revealed that Russia was “deeply divided over the pace and direction of economic reform, the role and rights of Russia in the ‘Near Abroad,’” and how fast to “integrate with the West” at all.
Lake warns Clinton that Russian reform faces the criticism that “average Russians” are “worse off than when the USSR collapsed two years ago.” So the U.S. would have a twofold message both encouraging Yeltsin to continue privatization and macro reform and understanding the need for “greater targeted social investments,” even though the U.S. cannot design or fully fund those—“that is clearly Russia’s job.”
On the hopeful side, the Moscow trip is taking place in the middle of an intensive three-way diplomatic process with Ukraine, working out compensation from Russia for the rapidly wasting nuclear warheads left over in Ukraine from the Soviet Union—all targeted on the U.S. but reaching the end of their working lives. Lake tells Clinton that a successful resolution here “would be the crowning achievement of the summit, a victory for your nonproliferation policy” and “a strong public symbol of Russia’s willingness to work fairly with its most important neighbor.”

U.S. Department of State, National Security Archive FOIA
This 12-page “eyes only” memo to the Secretary of State is the first in a long series of candid briefing memos written by Strobe Talbott in his role as the number two official in the State Department. Written just four days after Talbott had been formally nominated to be Deputy Secretary, the memo leaves a wide margin for Christopher’s reactions, and asks if he wants more such frank missives. The Secretary scribbles “yes” on the first page and adds many more comments on other pages. Talbott displays the lively writing style developed in his previous two-decade tenure at TIME magazine, and captures Christopher’s attention with colorful details on personalities, on the administration’s critics and how to disarm them, on the internal policy conflicts, and with constructive suggestions for ways forward. While recognizing that the new deputy secretary role would involve many more responsibilities than just U.S.-Russia policy, Talbott makes sure to impress upon Christopher that Clinton himself wanted Talbott’s continuing close engagement on Russia, especially for Clinton-Yeltsin meetings such as the Moscow summit that month.

In this memo to the national security adviser on the eve of Clinton’s visit to Moscow, Talbott previews some of the most important issues the Russia side wants to raise during the summit—the future security arrangements in Europe. Talbott writes quite dismissively and negatively about a new European security initiative that Russian Foreign Minister Andrey Kozyrev presented in a German newspaper, which he called the “Partnership for United Europe.” The plan would subordinate NATO to the CSCE structures and strengthen the Russian role in building a new integrated Europe. Although the Clinton team stated publicly that a fully integrated Europe without new dividing lines was their goal, Talbott dismisses Kozyrev’s thoughts on Russian desire to “be the architect […] along with the U.S. of a completely new European security order,” saying that “it sticks in their craw that NATO appears poised to dictate the terms of the new order.”
Talbott’s early relationship with Kozyrev had been cordial and productive, but now his view of Kozyrev has changed completely. He sees the Russian foreign minister moving in a more nationalist direction partly as a result of the December elections and his own political interests. Talbott concludes that “Kozyrev has become part of the problem rather than part of the solution” and suspects that he was an unhelpful influence on Yeltsin during the last weeks of the trilateral process. He shares with Lake the talking points for Secretary Christopher’s upcoming meeting with Kozyrev where they were scheduled to discuss the Partnership for Peace and European security.

Strobe Talbott’s backstage version of this conversation, published in 2002, gave a colorful, conflict-ridden account of the Clinton stopover in Kyiv on the way to Moscow. But that version is not supported by the actual transcript, only declassified in 2018. According to Talbott, “Clinton and Christopher, neither of whom was in the habit of roughing up a head of state, decided to make an exception. They told Kravchuk in the bluntest of terms that if he backed out of the deal that had already been made it would be a major setback for Ukraine’s relations with both Russia and the U.S.” Kravchuk was “visibly shaken” in Talbott’s version.
The transcript (Talbott was not actually in the room, according to the list of participants on the memcon) shows far more diplomatic language, with Clinton praising Kravchuk’s “enormous vision and courage” and promising “to do everything I can for the people of Ukraine and for you, sir.” Clinton points to the $175 million in Nunn-Lugar funds coming to underwrite the nuclear dismantling and offers to persuade the G-7 and the IMF to develop ways to pay for energy imports (Ukraine’s were all from Russia and already indebted).
Kravchuk responds gratefully: “Certainly we should start our broader cooperation so that I can tell our people that after I took this position on the nuclear question, there was a change in attitude toward assistance to our country. That would help. When we have stabilization for our currency and private investment for Ukraine, then everyone will understand that the agreements signed by the three Presidents were the only possible step.” The only apparent moment of U.S. pressure comes when Christopher says the signing in Moscow will be “an historic event and a celebration. It will not be a negotiating session.” The conversation concludes with interesting commentary by Kravchuk on Russia to the effect that Yeltsin understands, “but there is no eternal president and we worry about expansion.”

While Yeltsin is eager to greet Bill Clinton in the Kremlin, the long-awaited state visit is happening in less-than-ideal circumstances. The December Duma elections brought a backlash against the democratic forces due to the harshness of economic reform and Yeltsin’s heavy-handed approach to the constitutional crisis. Clinton’s planned speech to the newly elected parliament had to be scrapped. Yeltsin starts the meeting talking about the composition of the new Duma saying that he does not “share the concern that is felt abroad about Zhirinovskiy.” Yeltsin gives a correct diagnosis that the Zhirinovsky vote was a response to economic hardship, that the people “didn’t vote for taking back Alaska, Ukraine and Crimea or for the fascism that he embodies but rather because they are unhappy.”
In response, and clearly in view of the future presidential election in Russia, Clinton gives his counterpart some sound political advice on how to work with the opponents and how to make his political program more appealing to people. He says that “the reformers’ campaign showed a recklessness,” comparing it to some U.S. Democrats. Clinton advises Yeltsin to come back to the image of the man on the tank and focus more on values, not programs. He offers Yeltsin advice from his political experts, suggesting he should send Gaidar or his other associates to Washington for a “quiet meeting,” which Yeltsin enthusiastically accepts.
Turning to economic reform, Yeltsin complains about the slow pace of G-7 and IMF assistance and the lack of U.S. investment in Russia and points to the continued existence of the Jackson-Vanik amendment. He says Russia does not “want aid since that can lead to an anti-Western flair-up.” He asks for investment, help in rescheduling of foreign debt, and to redirect 10% of the Nunn-Lugar program funding to Russian research institutes. Clinton expresses his continued support for the reform but names three issues that prevent him from moving more decisively in his Russia agenda— Russia’s arms sales to Iran, slowness in joining the Partnership for Peace, and lack of agreement on the withdrawal of Russian troops from the Baltic states.

This document is a placeholder for the first big economic discussion of the summit, the “First Expanded Bilateral” that took place on the morning of January 13, 1994. The highly professional staff at the Clinton Presidential Library have to date been unable to locate a transcript (memcon) of this meeting and speculate that perhaps one was not written up afterwards, perhaps due to a division of labor between the National Security Council staff, who would normally have taken the notes, and the Treasury Department personnel, who were also in attendance and were personally invested in the Yeltsin economic reform program.
Beforehand, the NSC and Treasury staff prepared Clinton for the meeting with this revealing briefing memo and talking points. The memo says, “The Parliamentary elections were a wake-up call generally, but specifically the Russian people view the government’s two-year attempt to begin a historic economic transition from command economics to a ‘Russian’ market economy as harsh and directly responsible for the decline in living standards during this period. Russia’s economic reforms have not succeeded on at least two fronts. They haven’t established a social safety net for the average Russian and have also not reduced subsidies to large state enterprises.”
Clinton’s main concern was to insist on continuing “bold economic reforms” while acknowledging “our understanding” of “greater emphasis on social welfare programs.” At the top of Clinton’s talking points was the affirmation “that reform program and team will stay in place.” That was not to be. In fact, during the summit the Americans learned that the leading reformers, Yegor Gaidar and Boris Fyodorov, were being fired by Yeltsin. Perhaps the Americans shouldn’t have been so surprised. Even Strobe Talbott had commented in December that the U.S. goal now after the elections was to promote “less shock and more therapy.” The remark “managed to infuriate both Russian liberals and my colleagues at Treasury,” Talbott later wrote; “it sounded to both groups that I too was blaming Gaidar and Fyodorov for the rise of Zhirinovsky and undercutting our own government’s insistence on rapid, disciplined structural reform and strict conditionality for IMF lending.” [6] Indeed.

President Clinton meets with the Russian Orthodox Patriarch in the hospital as part of the plan to widen his circle of Russian interlocutors and to appeal to the Russian believers. Clinton’s mother has just passed, and the Patriarch offers a prayer for her. Aleksiy expresses his church’s support for democratic reform in Russia and his concern about the results of the recent parliamentary elections. He delicately hints about the proliferation of various proselytizing preachers from the West, who flooded Russia in the early 1990s, which the official Orthodox church saw as a competing influence on Russians’ souls. Clinton responds that he is a Baptist himself (a denomination previously considered a sect in the USSR) and that he appreciates the religious liberty in Russia. He reminisces about his visit to the Novodevichy Monastery 24 years ago and his visit to St. Basil’s Cathedral earlier in the day. The U.S. president’s remarks are very brief but empathetic and respectful of Russian spirituality. It’s worth noting that the second Church official in attendance, Metropolitan Kirill, went on to become the Patriarch himself, blessing the Russian invasion of Ukraine in 2022.

The dinner at Yeltsin’s official dacha in Novo-Ogarevo features moose lips in wine sauce, Bill Clinton playing saxophone, and a most lavish 24-course dinner with lots of toasts. It was designed as a showcase of the U.S.-Russian partnership and a display of warm personal relationship between the two presidents. The cable drafted by Ambassador Pickering misdates the dinner, which took place on the evening of January 13. Among the many issues covered, most important were the Russian role in European security, the Partnership for Peace and the future expansion of NATO, and Russian policies in the near abroad.
Yeltsin reflects on the challenges of working with the new parliament and the changes he was planning to make in his cabinet. Clinton comes to the dinner straight from the Spaso House reception where he met with representatives of many Russian political parties and movements, most of whom were critical of Yeltsin’s policies. Yeltsin gives his counterpart a somewhat optimistic review of his prospects of working productively with the new parliament but mentions that Yegor Gaidar would have to step down and work in the Duma.
Turning to the security agenda, Yeltsin tells Clinton that his information about arms sales to Iran is incorrect and asks him if sanctions on Iraq could be eased so that Russia could collect some of the debt that Iraq still owed it. Clinton noted that if Iraq was permitted to sell oil, the falling oil prices would harm Russian interests. Defense Minister Grachev talks about military-to-military relations, his recent meetings with U.S. Defense Secretary Les Aspin, and his first call on the Partnership for Peace hotline on January 5, 1995. He wants to meet with the new U.S. Defense Secretary as soon as possible (retired Admiral Bobby Inman had been nominated by Clinton to succeed Aspin but later withdrew) and to brief the Secretary General of NATO on the new Russian military doctrine. Grachev is very pleased with the close cooperation with the U.S. military and even invites Clinton and Yeltsin to personally observe a planned bilateral military exercise in July 1994.
One of most important issues for the U.S. team, according to the scene-setter, is the deployment of Russian peace-keeping forces in the near abroad. This issue is painful for Yeltsin, who is trying to be a force for good in the former Soviet space. The Russian president talks about Russia’s constructive actions in Moldova and Georgia and his desire to stop bloodshed. He says that “allegations of imperial aspirations are harming us and are not correct.”
Yeltsin wants to speak about his favorite subject—U.S.-Russian partnership, and Russia’s relationship with NATO. In his memoir, Kozyrev wrote that Yeltsin was shocked by Clinton’s “not whether but when” statement in Prague about future NATO expansion, and even felt betrayed by Clinton. Here, however, Yeltsin says to Clinton “we certainly agree with you on NATO” but also states that “Russia has to be the first country to join NATO,” followed by other states from Central and Eastern Europe. He even proposes “a kind of cartel of the U.S., Russia and Europeans to help to ensure and improve world security.” Clinton’s response is very careful, mentioning Russian’s sense of greatness but not engaging on the idea of a cartel or Russia’s membership in NATO.
The Russian president expresses his deep appreciation of Clinton: “You come to Russia not to confront us, but with the affection and love of our people and with a sense of support for Russia.” In response, Clinton talks about their “relationship of trust and confidence” and the unique chance it creates if Russia stays the course: “we could guarantee the countries of Europe a century of peace or more.” Such were the high hopes of the 1990s.

This very brief (one might even say rushed) meeting between presidents Clinton, Kravchuk and Yeltsin is the formal event to present the “crowning achievement” of the summit—the Trilateral Statement on withdrawal of nuclear weapons from Ukraine. This meeting was preceded by months of work by diplomats from all three countries and was still in question as Clinton was flying to Europe. Yeltsin summarizes the details of the statement, emphasizing that “Russia and the U.S. will give [Ukraine] full guarantees of security.” Kravchuk confirms that “there is no alternative to nuclear disarmament” and pledges full cooperation with the trilateral process. Clinton commends Russia and Ukraine on their cooperation and praises the Trilateral Statement that “this agreement makes the world safer and each of our countries more secure.” Relieved that the agreement was finally in hand, he suggests that everybody has already said enough and they “should go sign the agreement.” Yeltsin suggests they exchange the letters spelling out each side’s commitments first.

Document 10
Clinton Presidential Library
In his scene setter (Document 1) Tony Lake calls the prospective signing of the Trilateral Statement “the crowning achievement of the summit.” The U.S. team doubted that they would be able to sign it with the agreed wording even as they arrived in Moscow. Both the Ukrainians and the Russians were trying to reopen the text, but Clinton pushed back on both. [7] The historic agreement achieves the goal of Ukrainian nuclear disarmament as well as settling the issue of Russia’s payment for the uranium contained in the nuclear warheads that would be moved to Russia for dismantlement. Ukraine commits to eliminating all nuclear weapons on its territory by dismantling them and sending the warheads to Russia. Russia commits to providing Ukraine with fuel rods for civilian nuclear power plants using downgraded uranium from the warheads. The United States commits to providing assistance for dismantlement of nuclear weapons under the Nunn-Lugar Program. Ukraine is to get at least $175 million of this assistance. All three signators commit to treating each other as full and equal partners. The Annex specifies the security assurances that Russia and the United States gave Ukraine (these were later formalized in the Budapest Memorandum in December 1994). However, the assurances did not go beyond the commitments already contained in the text of the Helsinki Final Act and the Non-Proliferation Treaty. Russia violated its commitments in 2014 with the annexation of Crimea and again with the invasion of Ukraine in 2022.

Document 11
After the Trilateral Statement is signed, the entire Russian and U.S. security teams meet face to face to discuss dozens of issues on their agenda. Clinton starts by noting that the HEU agreement first discussed in Vancouver, under which the United Stated would purchase the uranium from nuclear warheads dismantled in Russia, is being signed later in the day. After mentioning START I, the U.S. president moves quickly to raise a very sensitive issue—biological weapons remaining from the Soviet program. His talking points from Anthony Lake instruct him to link the issue to Nunn-Lugar certification if Russia does not resolve bio concerns. Clinton reminds Yeltsin of his personal commitment to end the program and notes that U.S. experts believe that illegal work was still happening. Yeltsin and Defense Minister both deny that anything illegal is going on and “shake [their] head[s] vigorously.”
In the first part of the conversation, Yeltsin seems not fully engaged, saying almost nothing, stating that Russia has ratified the Chemical Weapons Convention (it had not) and sounding surprised when Clinton mentions Russian arms sales to Iran, Libya and North Korea. However, when he takes the floor, he is quite eloquent but prefers to talk about grand ideas and designs rather than specific issues. His favorite subject is how the U.S.-Russian partnership would transform the world, creating a “new system of international relations.” He suggests they should “propose an initiative to reshape world institutions such as the United Nations and the Conference for Security and Cooperation in Europe.” He sees a need to formulate an official document formalizing the U.S.-Russian partnership, sounding like a partnership between two superpowers (even while Russia, by 1994, was just a shadow of the former USSR). While Yeltsin wants to reshape the world and build new structures, the U.S. side is concerned about preserving and expanding its own main security structure—NATO. Clinton wants to be very careful about how they describe the partnership, so that other countries “don’t think we are dividing Europe.” Clinton expresses his commitment to a fully integrated Europe “for the first time in history” but does not engage with Yeltsin on his grand reformist designs.
The meeting is very productive in terms of achieving specific understandings on ABM, the need to ratify START II, Soviet troops in the Baltics and elimination of chemical weapons. Russian Defense Minister Pavel Grachev carries most of the arms-control and non-proliferation discussions for the Russian side.

Document 12
This last conversation was not planned, but Clinton requested it after he learned that Yeltsin was about to let go of some of his most committed reformers led by Yegor Gaidar, who would resign the day after the U.S. delegation left Moscow. Clinton (who does all the talking here) tells Yeltsin that he now understands better that Russian people were hurt by the reform, that “most ordinary citizens and some well-educated ones did not feel connected to what Yeltsin was doing,” and that they “did not feel that their lives had improved.” Still, he encouraged Yeltsin to try to keep the reformist team, among other things, because Clinton’s “credibility was connected to President Yeltsin’s indication that he would continue the reforms, which were linked to a specific team of people.” Departure of those people would hurt Clinton’s ability to deliver on promises of IMF credits and debt relief. Yeltsin replies only that “President Clinton saw the situation, and these difficulties did exist.”
[1] The most revealing insider account of U.S.-Russia policy in the 1990s is found in Strobe Talbott’s The Russia Hand (2002). The title refers not to Talbott himself, but rather to Bill Clinton, who personalized his support for Boris Yeltsin all the way to Yeltsin’s resignation in 1999, anointing his successor, Vladimir Putin.
[2] The Ostankino event with young people is the highlight of Clinton’s own account of the summit in his book, My Life (2004, pp. 570-71), while Talbott commented: “Watching Clinton prepare for and deliver this speech was both frightening and inspiring, in that it captured both his indiscipline and his genius,” scribbling amendments to his speech text in the car on the way. ( The Russia Hand , p. 115)
[3] The most thoughtful high-level Ukrainian account is in Volodymyr Horbulin, “Nuclear Disarmament of Ukraine,” pp. 240-254 of his memoir, My Journey In The Looking Glass (2019), translated by Sarah Dunn in the National Security Archive e-book, Nuclear Weapons and Ukraine , https://nsarchive.gwu.edu/briefing-book/nunn-lugar-russia-programs/2019-12-05/nuclear-weapons-ukraine . A rocket engineer, Horbulin served as Ukraine’s national security adviser and as head of the Ukrainian Academy of Sciences.
[4] The blow-by-blow of the extensive diplomacy involved, both bilaterally with Ukraine and trilaterally including Russia, is detailed in Steven Pifer, The Eagle and the Trident (2017), pp. 37-76, with reflections on this history post-Crimea. The best scholarly account is in Mariana Budjeryn, Inheriting the Bomb (2023).
[5] For the backstage story, see Strobe Talbott, The Russia Hand , pp. 117-118, including the commentary from Talbott’s counterpart, Yuri Mamedov: “You want us to be a democracy, so don’t be surprised when a president and a prime minister have to sacrifice a minister or two who are tarred with the brush of failed policies. This is real politics. At least we don’t shoot people.” For an indictment of the Gaidar program, see Peter Reddaway and Dmitri Glinski, The Tragedy of Russia’s Reforms (2001). For an invaluable oral history centering on Gaidar, see Petr Aven and Alfred Kokh, Gaidar’s Revolution (2015).
[6] See Strobe Talbott, The Russia Hand , pp. 106-107.
[7] For the details of Clinton’s pressure both on Kravchuk and on Yeltsin, see Strobe Talbott, The Russia Hand , pp. 112-113.
Middle East latest: Israeli foreign ministry outlines 'initial price' Iran must pay for missile attacks - as Tehran warns of 'bigger' retaliation
Israeli military spokesman Rear Admiral Daniel Hagari said aircraft are still patrolling the skies. Meanwhile, President Joe Biden said the US military helped Israel "take down nearly all of the drones and missiles" fired by Iran.
Sunday 14 April 2024 15:07, UK
- Israel-Hamas war
Please use Chrome browser for a more accessible video player
- More than 300 drones and missiles fired on Israel by Iran, says IDF
- Iran threatens US bases and larger attack on Israel if it retaliates
- Gantz: 'Event not over' and Israel 'will collect a price'
- Israel outlines 'initial price' Iran must pay
- RAF shot down Iranian attack drones
- Michael Clarke analysis : Why it's likely not the end of cycle of violence
- Explained: Everything we know so far about the attack
- Sean Bell analysis: This could have been much worse - the challenge is now Netanyahu's reaction
- Live reporting by Ollie Cooper and Brad Young
Joe Biden has told Benjamin Netanyahu the US would not participate in any Israeli counterattack against Iran, according to reports.
CNN and the Wall Street Journal cited senior US officials as saying Mr Biden told the Israeli prime minister a response was unnecessary.
The US will continue to help Israel defend itself, but does not want war with Iran, White House national security spokesperson John Kirby told ABC News.
"We don't seek escalated tensions in the region. We don't seek a wider conflict," he said.
The Syrian foreign ministry has called Iran's attack a "legitimate right to self-defence".
Yemen's Houthis movement also said it was a legitimate act in response to a suspected Israeli strike on the Iranian consulate in Damascus on 1 April.
A Houthi spokesman added the group had been in direct confrontation with Israel since 7 October.
The Israeli minister for national security has said Israel must go "berserk" in response to Iran's attack.
Itamar Ben Gvir, a member of the war cabinet, said Israel "must not be wimpish".
"The concepts of containment and proportionality, these concepts became obsolete on 7 October.
"In order to establish deterrence in the Middle East, you must show them that you've gone berserk and completely lost it."
The first direct attack on Israel by Iran has shaken Israelis and left them fearful a bigger war is looming.
Iranian weapons and interceptors could be seen flashing over the sky at night.
"I think it was quite scary when in the middle of the night we started hearing booming and we didn't know what it was, I mean we knew what it was, we didn't know to what extent it would be," said Jerusalem resident Cecile Smulowitz.
"But thank God the Israeli army came through, and so far it's quiet and we hope it would continue that way."
Some Israelis said they did not want an escalation but with the stakes so high they were nervous.
"I really hope there won't be a big war, none of us in Israel wants a big war so I hope that's it, and I hope Iran would stop now," said Jeremy Smith, 60, a resident of Tzur Hadassah.
"I imagine Israel will respond because I mean, our whole country was covered in missiles and drones. So what can you do? But we have to stop it somehow."
Jerusalem resident Amy Friedlang Morgans, 71, said: "We don't want a war with Iran, somehow they can't accept Jewish people living here. This is our homeland, it's written in the Bible."
Some further Israeli reaction to last night's attacks by Iran, with war cabinet minister Benny Gantz promising a response.
Mr Gantz, himself a retired army general, said that Israel would exact a price from Iran in a manner and time "that's right for us".
"Yesterday, Iran launched an attack on Israel - and met the strength of the Israeli security system," he said in a statement - just ahead of an expected war cabinet meeting.
He described Iran as a "global problem" as well as a regional challenge.
"This event is not over - the strategic alliance and the regional cooperation system that we built and stood its significant test need to be strengthened precisely now," he added.
He said that Israel had proven itself "an anchor of military and technological power" and of security in the region.
"Faced with the threat of Iran - we will build a regional coalition and collect the price from Iran, in the way and at the time that suits us," he said.
Footage released by Iranian state TV reportedly shows missiles being launched from Iran.
Some of the more than 150 cruise and ballistic missiles are shown blasting into the sky, leaving smoke trails behind.
While a military base sustained light damage, only a "handful" of the more than 300 missiles and drones made it through Israel's air defences, according to the IDF.
The Israeli foreign ministry has outlined an "initial price" it says Iran must pay for its attack.
The Iranian Revolutionary Guards Corps must be recognised as a terrorist organisation, said spokesperson Lior Haiat.
"Painful sanctions" must also be imposed on Iran, including in the field of missiles, he added.
"Israel has the right to defend itself in the face of Iran's massive attack. Israel successfully defended itself against Iran's aggression and will continue to do so in the future," read a statement.
Iran's attack on Israel, its threats issued to the US and its supply of weapons to Russia "endangers world peace", said Mr Haiat.
"This is precisely why Iran must never obtain nuclear weapons," the statement said.
Israel's air defence system is one of the most effective in the world.
The system consists of a series of truck-towed mobile units placed strategically throughout the country.
When their radars detect a threat, the information is sent to a 'battle management centre' where military personnel analyse it, anticipating its path and impact point, and decide which missile launcher to use to intercept it.
Read on here...
Italy, which holds the presidency at the G7, has confirmed our earlier reports that it will hold a meeting of the group this afternoon.
In a short statement, the Italian presidency website said the remote meeting would be held early this afternoon to "discuss Iran's attack against Israel".
The US, UK, Canada, France, Germany, Italy and Japan make up the members of the Group of Seven major economies, set up in 1973.
Iran's actions, like Russia's, threaten to cause a larger conflict, Ukrainian president Volodymyr Zelenskyy has said.
He called for efforts to prevent further escalation in the Middle East.
"Iran's actions threaten the entire region and the world, just as Russia's actions threaten a larger conflict.
"The obvious collaboration between the two regimes in spreading terror must face a resolute and united response from the world."
Be the first to get Breaking News
Install the Sky News app for free


IMAGES
VIDEO
COMMENTS
For applicable clinical trials, basic results must be posted within 12 months of the Last Patient Last Visit (LPLV) for the primary endpoint. If the primary endpoint occurs before the end of the study, then basic results must be posted twice, once within 12 months of the primary endpoint LPLV and again within 12 months of the study LPLV.
First Patient In (FPI) is a key indicator of the overall success of a study. If that first patient is enrolled on time or within 30 days of the FPI goal, your study will begin on sound footing. ... (Last Patient/First Visit) goals. In the past, about 70 percent of our business consisted of rescue programs for low-enrolling studies. We have seen ...
Primary outcome measures of this study were the visit and injection numbers during the first year. Results: Eight hundred eighty eyes of 783 patients met the inclusion criteria for the study.
Last patient, First visit; 3. Last patient, Last visit; 4. Clinical study report) 3. Overall study plan. In addition to the scope, objectives, milestones and timelines, the overall study plan should include the study budget and the resource plan.
On average, phase II/III oncology protocols are 1.5 times longer than non-oncology protocols with the widest differences observed in durations associated with patient enrollment. Completion rate was also substantially lower for oncology protocols than for non-oncology protocols—31.4% and 80.0%, respectively.
Conducting clinical trial feasibility is one of the first steps in clinical trial conduct. This process includes assessing internal and environmental capacity, alignment of the clinical trial in terms of study design, dose of investigational product, comparator, patient type, with the local environment and assessing potential of conducting clinical trial in a specific country.
The duration to complete all first patient visits (First Patient First Visit—Last Patient First Visit), the overall treatment duration (First Patient First Visit—Last Patient Last Visit), and the total clinical trial duration from protocol approval to database lock showed weak but significant positive correlations (0.283, 0.342, and 0.323 ...
FPFV, first patient first visit; LPFV, last patient first visit; LPLV, last patient last visit from publication: Design and rationale of a large, international, prospective cohort study to ...
b) Projected Last Patient/Last Visit 1. LPLV at "X" 4. Close-out Visits Completed* The projected date for next IMV Number of pages to be verified by CRA at next IMV Number of days CRA spends at site Project date of last patient/last visit at the site. This date is projected by using the visit windows in the protocol
The duration to complete all first patient visits (First Patient First Visit—Last Patient First Visit), the overall treatment duration (First Patient First Visit—Last Patient Last Visit), and the total clinical trial duration from protocol approval to database lock showed weak but significant positive correlations (0.283, 0.342, and 0.323 ...
Background Benchmark data characterizing protocol design practices and performance informs clinical trial design decisions and serves as important baseline measures for assessing protocol design behaviors and their impact during and post-pandemic. Methods Tufts CSDD, in collaboration with a working group of 20 major and mid-sized pharmaceutical companies and CROs, gathered phase I-III data ...
Definition: Date when the last participant completes a clinical trial. Although LPLV is often used synonymously with study completion, the latter may still include measurements and data collection without face-to-face involvement between a participant and an investigator.
Inventiva anticipates the last patient first visit for the NATiV3 clinical trial in the first half of 2024. About lanifibranor. Lanifibranor, Inventiva's lead product candidate, is an orally-available small molecule that acts to induce antifibrotic, anti-inflammatory and beneficial vascular and metabolic changes in the body by activating all ...
The last patient first visit in the NATiV3 Phase III clinical trial is now expected in the first quarter of 2024, and the target number of randomized patients in the main cohort is expected to be reached in the second quarter of 2024. Analysis of the baseline characteristics of all patients randomized in the main
First Patient First Visit; Capture subject accruals; Report into Documas; Monitor trial; Review amendments; Undertake financial management; Last Patient Last Visit; Close site; Reporting; Archiving and publication; Stakeholders. Clinical Divisions; Research Governance and Integrity Team; Pharmacy; Imaging research; Tissue Bank; Pathology ...
The duration to complete all first-patient visits (first patient first visit to last patient first visit), the overall treatment duration (first patient first visit to last patient last visit), and the total clinical trial duration from protocol approval to database lock showed weak but significant positive correlations with the number of ...
The pause will likely "extend the last patient first visit timeline for NATiV3 trial to the first half of 2024," the biotech noted in its announcement. Prior to the pause, NATiV3 was on track to complete screening in the first quarter of 2024.
A Phase 1 Dose Escalation and Expanded Cohort Study Of PF-06821497 In The Treatment Of Adult Patients With Relapsed/Refractory Small Cell Lung Cancer (SCLC), Castration Resistant Prostate Cancer (CRPC) And Follicular Lymphoma (FL). ... approximately 2 years past last patient first visit. ] Objective response using Response Evaluation Criteria ...
The study's final patient follow-up visit was March 12, 2022. This randomized, double-blind trial is the first systematic, placebo-controlled, single ascending dose study to investigate very low ...
President Nixon returned to the United States on May 30. Nixon's visit to Moscow on this day in 1972 was a step toward conciliation (in the form of space cooperation and the signing of the SALT arms control treaty) in the depths of the Cold War. Today, the United States and Russia may be over two decades removed from the Cold War, but there ...
EPIC MOSCOW Itinerary! (2024) Moscow is the heart of Mother Russia. Just the mention of this city conjures images of colorful bulbous pointed domes, crisp temperatures, and a uniquely original spirit! Moscow has an incredibly turbulent history, a seemingly resilient culture, and a unique enchantment that pulls countless tourists to the city ...
The last patient first visit in the NATiV3 Phase III clinical trial is now expected in the first quarter of 2024, and the target number of randomized patients in the main cohort is expected to be ...
Moms and dads can prepare, too. When making the appointment, it can't hurt to ask for any necessary patient forms ahead of time. It may be quicker and easier for you to fill them out at home instead of at the office on the day of your visit. Make a list of questions, as well.
Washington, D.C., January 25, 2024 - Declassified highest-level records from the Moscow summit 30 years ago this month detail U.S. President Bill Clinton's strong personal support for Russian President Boris Yeltsin, their close cooperation on security issues, and deep concern about Yeltsin backtracking on economic reforms newly understood by the Clinton team as too "harsh" on the ...
The Israeli military said earlier that more than 200 drones and missiles have been launched by Iran since last night. 03:54:11 Biden: 'I condemn these attacks in the strongest possible terms'