National Aeronautics and Space Administration
Goddard space flight center, imagine the universe, astronomer's toolbox.
- Cosmic Objects
- Big Questions
- Featured Science
- Observatories
- Scientist Profiles
- You Be the Astrophysicist
- The Cosmic Distance Scale
- Lesson Plans
- Ask an Astrophysicist
- Other Resources
- News #include virtual="/news/newsNav.html"
Additional Links
For Educators
Electromagnetic Spectrum - Introduction
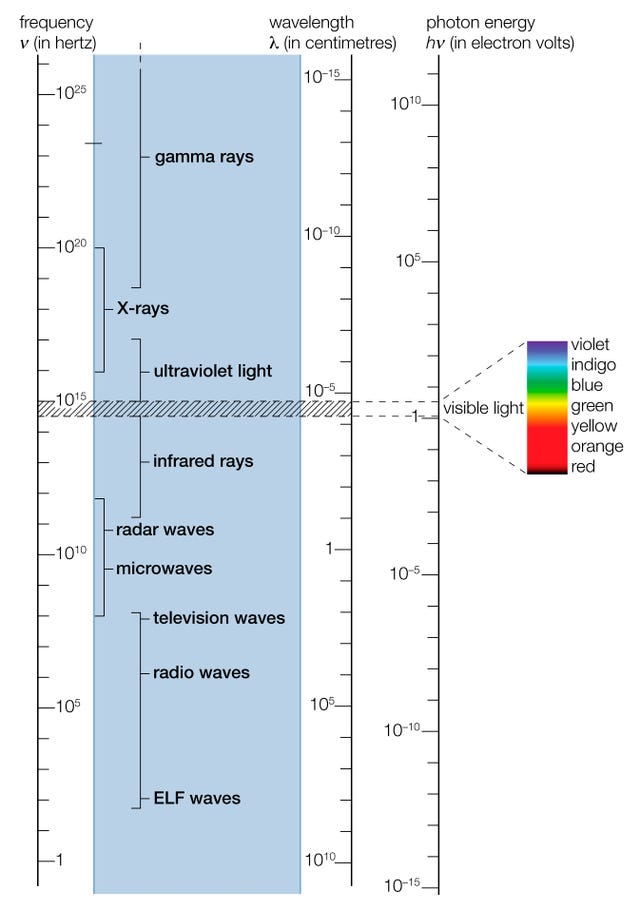
The electromagnetic (EM) spectrum is the range of all types of EM radiation . Radiation is energy that travels and spreads out as it goes – the visible light that comes from a lamp in your house and the radio waves that come from a radio station are two types of electromagnetic radiation. The other types of EM radiation that make up the electromagnetic spectrum are microwaves , infrared light , ultraviolet light , X-rays and gamma-rays .
You know more about the electromagnetic spectrum than you may think. The image below shows where you might encounter each portion of the EM spectrum in your day-to-day life.
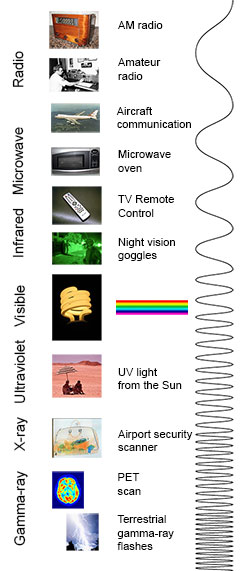
The electromagnetic spectrum from lowest energy/longest wavelength (at the top) to highest energy/shortest wavelength (at the bottom). (Credit: NASA's Imagine the Universe)
Radio: Your radio captures radio waves emitted by radio stations, bringing your favorite tunes. Radio waves are also emitted by stars and gases in space.
Microwave: Microwave radiation will cook your popcorn in just a few minutes, but is also used by astronomers to learn about the structure of nearby galaxies .
Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars.
Visible: Our eyes detect visible light . Fireflies, light bulbs, and stars all emit visible light.
Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well.
X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays.
Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe.
Is a radio wave the same as a gamma ray?
Are radio waves completely different physical objects than gamma-rays? They are produced in different processes and are detected in different ways, but they are not fundamentally different. Radio waves, gamma-rays, visible light, and all the other parts of the electromagnetic spectrum are electromagnetic radiation.
Electromagnetic radiation can be described in terms of a stream of mass-less particles, called photons , each traveling in a wave-like pattern at the speed of light . Each photon contains a certain amount of energy. The different types of radiation are defined by the the amount of energy found in the photons. Radio waves have photons with low energies, microwave photons have a little more energy than radio waves, infrared photons have still more, then visible, ultraviolet, X-rays, and, the most energetic of all, gamma-rays.
Measuring electromagnetic radiation
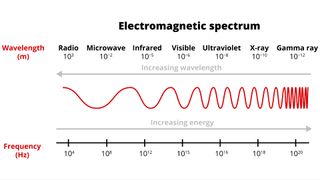
Electromagnetic radiation can be expressed in terms of energy, wavelength, or frequency . Frequency is measured in cycles per second, or Hertz . Wavelength is measured in meters . Energy is measured in electron volts . Each of these three quantities for describing EM radiation are related to each other in a precise mathematical way. But why have three ways of describing things, each with a different set of physical units?
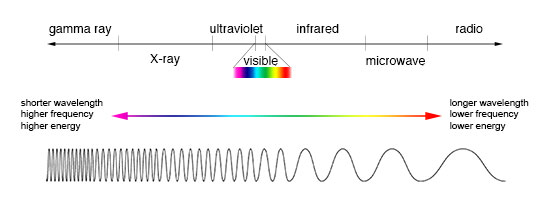
Comparison of wavelength, frequency and energy for the electromagnetic spectrum. (Credit: NASA's Imagine the Universe)
The short answer is that scientists don't like to use numbers any bigger or smaller than they have to. It is much easier to say or write "two kilometers" than "two thousand meters." Generally, scientists use whatever units are easiest for the type of EM radiation they work with.
Astronomers who study radio waves tend to use wavelengths or frequencies. Most of the radio part of the EM spectrum falls in the range from about 1 cm to 1 km, which is 30 gigahertz (GHz) to 300 kilohertz (kHz) in frequencies. The radio is a very broad part of the EM spectrum.
Infrared and optical astronomers generally use wavelength. Infrared astronomers use microns (millionths of a meter) for wavelengths, so their part of the EM spectrum falls in the range of 1 to 100 microns. Optical astronomers use both angstroms (0.00000001 cm, or 10 -8 cm ) and nanometers (0.0000001 cm, or 10 -7 cm ). Using nanometers, violet, blue, green, yellow, orange, and red light have wavelengths between 400 and 700 nanometers. (This range is just a tiny part of the entire EM spectrum, so the light our eyes can see is just a little fraction of all the EM radiation around us.)
The wavelengths of ultraviolet, X-ray, and gamma-ray regions of the EM spectrum are very small. Instead of using wavelengths, astronomers that study these portions of the EM spectrum usually refer to these photons by their energies, measured in electron volts (eV). Ultraviolet radiation falls in the range from a few electron volts to about 100 eV. X-ray photons have energies in the range 100 eV to 100,000 eV (or 100 keV). Gamma-rays then are all the photons with energies greater than 100 keV.
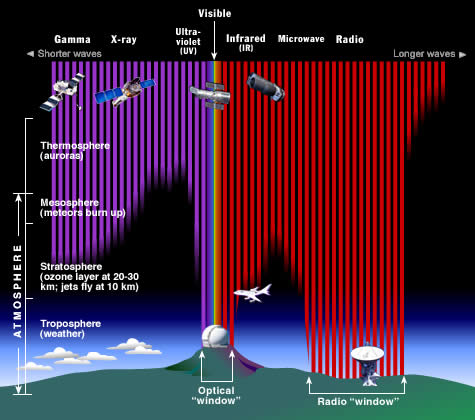
The Earth's atmosphere stops most types of electromagnetic radiation from space from reaching Earth's surface. This illustration shows how far into the atmosphere different parts of the EM spectrum can go before being absorbed. Only portions of radio and visible light reach the surface. (Credit: STScI/JHU/NASA)
Most electromagnetic radiation from space is unable to reach the surface of the Earth. Radio frequencies, visible light and some ultraviolet light makes it to sea level. Astronomers can observe some infrared wavelengths by putting telescopes on mountain tops. Balloon experiments can reach 35 km above the surface and can operate for months. Rocket flights can take instruments all the way above the Earth's atmosphere, but only for a few minutes before they fall back to Earth.
For long-term observations, however, it is best to have your detector on an orbiting satellite and get above it all!

- Project Leader: Dr. Barbara Mattson
- Web Curator: J.D. Myers
- Responsible NASA Official : Dr. Andy Ptak
- Privacy Policy & Important Notices
- Page Last Updated: 14-Nov-2014
The electromagnetic spectrum
Light, or electromagnetic radiation, comes in many forms. There are radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays and gamma rays, all of which form what is known as the 'electromagnetic spectrum'
The electromagnetic spectrum is subdivided into seven regions according to wavelength. Each portion of the spectrum interacts with matter in a slightly different way and is given a different name. From longest to shortest wavelength the seven divisions are:
Radio (wavelengths greater than 0.3 metres)
Earth's atmosphere hides most electromagnetic radiation from space except visible light, certain infrared frequencies and radio waves. For this reason, we can place radio telescopes on Earth's surface and radio astronomy was the first non-optical study of radiation from space. A number of the most massive galaxies were found to be extremely powerful sources of radio waves. Radio astronomy led to the discovery of pulsars which pulse regular radio emissions.
Microwaves (wavelengths between 1 millimetre and 0.3 metres)
Earth's atmosphere begins to shield radiation from us. The most important form of microwave radiation in astronomy is called the Cosmic Microwave Background (CMB). Discovered in 1965, CMB comes from all parts of the Universe with the same intensity. CMB became solid evidence for the 'Big Bang' theory, which predicted that the shockwave of the primeval explosion would be still detectable. ESA's Planck mission will study the CMB and thus will be seeing the Universe as it was almost at its beginning.
Infrared (wavelengths between 700 nanometres – 1 millimetre)
The primary source of infrared radiation is heat. The higher the temperature, the faster the atoms and molecules in an object move and the more infrared radiation. The first infrared space mission was IRAS (Infrared Astronomical Satellite) which detected about 350 000 infrared sources. Later, ESA's Infrared Space Observatory (ISO) made important studies of the dusty regions of the Universe. ESA's Herschel mission will build on this work.
Visible (wavelengths between 400 – 700 nanometres)
Until 1945, most astronomy was optical. This meant studying a very small range of wavelengths. It is from these optical wavelengths that most people derive their picture of the Universe, dominated by bright stars and galaxies. Visible light is predominantly released by objects between 2000 and 10 000°C. The NASA/ESA Hubble Space Telescope has a powerful optical telescope on board which enables it to take stunning photographs in real colour.
Ultraviolet (wavelengths between 10 – 400 nanometres)
As soon as observations from above the atmosphere became possible, the classical techniques of optical astronomy were extended into the ultraviolet. The Sun and other hot objects are sources of ultraviolet radiation. In 1978, the International Ultraviolet Explorer (IUE) was launched. IUE dominated ultraviolet space astronomy for nearly two decades. It generated spectra showing intensities at different wavelengths from selected objects in the sky. Temperatures, motions, magnetism and chemical composition are all discernible in the ultraviolet spectra.
X-rays (wavelengths between 0.01 – 10 nanometres)
Most of the observable matter in the Universe today is in a hot state, radiating short-wavelength radiation and X-rays. Massive clouds of gas at a very high temperature fill the spaces between galaxies. Whenever a new star is formed, a collapsing cloud of gas reaches temperatures sufficient for nuclear reactions to start, powering the star. Conditions in the primeval Universe were very different - with only a few pre-existing molecules and no dust available for cooling, only the most massive clouds could collapse. They would make not stars, but black holes. Theorists suspect that giant black holes may have been among the earliest objects created in the Universe and would have produced X-rays. Two ESA missions, XMM-Newton and XEUS are designed to observe these X-rays.
Gamma rays (wavelengths less than 0.01 nanometres)
Gamma rays from space are blocked by the Earth’s atmosphere – fortunately for us, because this powerful radiation is lethal. Gamma-ray telescopes in space give evidence for the processes that made the Universe habitable. When a massive star has used up its hydrogen fuel, it ends in a supernova explosion, emitting gamma rays. During this explosion, radioactive elements are formed and ejected into space, decaying or combining to form the other elements. ESA's COS-B satellite (1975-1982) created a catalogue of gamma-ray sources. ESA's Integral spacecraft, launched in 2002, takes this work forward, studying the phenomenon known as 'gamma-ray bursts'.
Thank you for liking
You have already liked this page, you can only like it once!
Related Links

ESA's gamma-ray astronomy mission
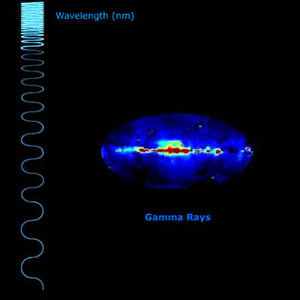
Seeing in gamma-ray wavelengths

Why do we observe gamma rays?
Gamma rays: Everything you need to know about these powerful packets of energy
Gamma rays can only be detected by sensors made of dense metals and takes over six feet (1.8 meters) of concrete to block.

How were gamma rays discovered?
How to stop gamma rays, are gamma rays dangerous, gamma-ray astronomy, gamma-ray bursts, additional resources.
Gamma rays are high-energy photons produced by some of the most violent events in the universe.
Photons of light are massless particles that are essentially packets of energy. Because of a quantum-mechanical phenomenon known as wave-particle duality , particles can behave like waves, and photons are no different. Photons have wavelengths, and the amplitude of their wavelength determines where they sit on the electromagnetic spectrum . Radio and microwave photons sit at the lower energy, longer wavelength end of the spectrum, while in the shorter wavelength, higher-energy regime are photons of ultraviolet, X-rays and the most energetic of them all with the shortest wavelengths: gamma rays.
Gamma rays have wavelengths shorter than 10^-11 meters and frequencies above 30 x 10^18 hertz. The European Space Agency describes how gamma-ray photons have energies in excess of 100,000 electronvolts (eV). We can compare this to X-rays, which NASA describes as having energies between 100 eV and 100,000 eV , and optical photons that we can see with our eyes, which are about 1 eV.
Related: What is the cosmic microwave background?
On Earth, gamma rays are produced by radioactive decay, nuclear weapons and lightning, while in space they are produced by violent, high-energy sources such as solar flares , quasars , black holes tearing stars apart , black-hole accretion disks, exploding stars and the strong gravitational environments of neutron stars .
At the turn of the twentieth century, two forms of radiation emitted by decaying atoms were known, namely alpha particles (which are helium nuclei) and beta particles (which are electrons and positrons).
However, when the French chemist Paul Villard began experimenting with the radioactive element radium, which had been discovered two years prior by Marie and Pierre Curie , he noticed that the ionizing radiation produced by the decay of radium packed a harder punch than either alpha or beta particles.
This radiation received its name — gamma-rays — simply because gamma is the third letter in the Greek alphabet after alpha and beta. Unbeknownst to Villard and his cohorts in the early 1900s, the key difference between gamma rays and alpha/beta particles is that gamma rays are a form of light, whereas alpha and beta particles are made of matter.
To block gamma rays requires a dense material, and the thickness of that material depends on the substance. To reduce the strength of incoming gamma rays by a billion, you need 13.8 feet (4.2 meters) of water, 6.6 feet (2 m) of concrete or 1.3 feet (0.39 m) of lead, according to the radiation protection solution website StemRad .
This poses a problem for gamma-ray telescopes, such as NASA's Fermi Space Telescope . Ordinary telescopes like the Hubble Space Telescope use mirrors and lenses to collect and focus light, but gamma rays will simply pass straight through an ordinary telescope. Instead, gamma-ray telescopes have to employ other means.
On the Fermi Space Telescope, a gamma-ray photon will pass through a device called the Anti-coincidence Detector, which blocks cosmic rays that might give a false signal, according to NASA . The gamma-ray is then absorbed by one of 16 sheets of tungsten, a material that is dense enough to stop gamma rays.
By interacting with the tungsten, the gamma-ray is converted into an electron and a positron (the antimatter or antiparticle counterpart of an electron), the paths of which are read by a tracker, which is a module of silicon strips interweaved by tungsten foil that can determine the direction that the gamma-ray came from in space, based on the trajectory of the electron and the positron.
Finally, the electron and then positron have their energies measured by a calorimeter — a device that measures the energy of a particle by absorbing it — made from cesium iodide, and therefore the energy of the gamma-ray can be determined.
Because of their high energy, gamma rays are ionizing, meaning they can dislodge electrons from atoms , ultimately damaging living cells and causing a hazard to health. However, as with all radiation, it depends upon the dose that you receive.
In small doses, very carefully targeted to limit exposure, they can be used safely as a medical diagnostic tool, or even to kill cancerous cells (ironic since exposure to radiation including gamma rays can cause cancer). In particular, one tool used by doctors is the ' Gamma Knife ', which is an ultra-precise form of surgery in which a beam of gamma rays cuts away diseased brain cells and can even penetrate deep into the brain without damaging the exterior lobes.

Given their ionizing power, it's fortunate that Earth's atmosphere is able to block gamma rays from space. For astronomers, however, that's unfortunate, because it means that to conduct gamma-ray astronomy observatories have to either be built on mountaintops where the atmosphere is thinner or sent into space.
The first gamma-ray space telescope was launched in 1961 on the NASA Explorer 11 satellite, but things didn't really begin to kick off until the late 1960s and early 1970s with a major finding, and it wasn't even an astronomical telescope that made the discovery.
Over the years there have been many observatories, both on the ground and in space, that have been designed to observe cosmic gamma-ray radiations. In 1990, NASA launched the Compton Gamma-Ray Observatory as the gamma-ray counterpart to the Hubble Space Telescope. The Compton Gamma-Ray Observatory explored the cosmos from 1991 until 2000. The aforementioned BeppoSAX was a joint Italian–Dutch mission that operated between 1996 and 2003, while NASA launched HETE-2 (the High-Energy Transient Explorer; HETE-1 had previously failed in orbit) that tracked down many GRBs between 2000 and 2008.
Currently, as of the end of 2022, several satellites , observatories and telescopes continue to conduct gamma-ray astronomy both on Earth and in space. NASA's Swift satellite , launched in 2004, combines both X-ray and gamma-ray observations, as does Italy's AGILE satellite launched in 2007. In 2002, the European Space Agency launched INTEGRAL , the International Gamma-Ray Astrophysics Laboratory.. The current most sophisticated gamma-ray space telescope is Fermi, which NASA launched in 2008.
Meanwhile, on the ground, there are several gamma-ray observatories including VERITAS (Very Energetic Radiation Imaging Telescope Array System) at the Fred Lawrence Whipple Observatory in Arizona and HESS (High Energy Stereoscopic System) in Namibia.

In 1963, the Soviet Union, the United Kingdom and the United States signed a nuclear test ban treaty that prohibited the world's superpowers from testing any nuclear devices in the atmosphere or in space. However, the U.S. was suspicious that the Soviet Union wouldn't adhere to the treaty, so they launched the Vela series of satellites to watch for any pulses of gamma-ray radiation that could be coming from secretive nuclear detonations. As it happened, gamma rays were detected, but from space: random blasts of powerful gamma-ray energy that seemed to be coming from all around the Earth. But how far away were these gamma-ray bursts ?
Related: Most powerful gamma-ray burst ever seen could help reveal how black holes are born
If these gamma-ray bursts, which are abbreviated to GRBs for short, were coming from our galaxy, then astronomers would detect them mostly in the plane of the Milky Way . Instead, they were spread all over the sky, it could mean only one of two things. Either they were very close, within our solar system , or they were very far away, beyond our galaxy. A special debate was even convened in 1995, echoing a similar ' Great Debate ' in 1920 between Harlow Shapley and Heber D. Curtis that discussed the size of our galaxy based on the distribution of globular clusters .
In the 1995 debate , chaired by Martin Rees, astronomer Bohdan Paczynski of Princeton University argued that GRBs came from very far away, while Donald Lamb of the University of Chicago reasoned that GRBs must be from close by because the energy required for them to be billions of light-years away would contravene the laws of physics.
Just two years later astronomers had their answer when the BeppoSAX satellite detected a gamma-ray burst that the William Herschel Telescope in the Canary Islands was able to quickly follow up on, in the process detecting the faint afterglow of whatever explosion had created the GRB. Measuring the redshift of the afterglow's light revealed it to have come from six billion light-years away. Bohdan Paczynski was right!
There are two main types of GRB. One type is called short GRBs which last just fractions of a second, while the other kind is known as the long GRBs, and can last many seconds up to an hour. Short GRBS are emitted during the merger of two neutron stars , while long GRBs are the death cries of rare, massive stars .
Physicists Andrew MacFadyen and Stan Woosley of the University of California, Santa Cruz, developed a model to explain how stars could explode and produce long GRBs without breaking the laws of physics. When a massive star with 50–100 times the mass of the sun reaches the end of its life, the star begins to collapse in on its core, and if the star is rotating fast enough, the energy within the collapsing layers rebounds off the core and is blasted out in two jets that move at almost the speed of light and blow the star apart. Charged particles within these jets spiral around powerful magnetic fields and produce something called synchrotron radiation, in the form of the gamma rays that we observe. Because the gamma rays are only released in the direction of the jets, and not in all directions at once, the total energy released does not contravene the laws of physics.
Learn more about ionizing radiation with the United States Environment Protection Agency (EPA) , and the American Cancer Society . Explore gamma rays in more detail in a tour of the electromagnetic spectrum with NASA Science.
Follow Keith Cooper on Twitter @21stCenturySETI. Follow us on Twitter @Spacedotcom and on Facebook .
Bibliography
Flash! The Hunt for the Biggest Explosions in the Universe by Govert Schilling (Cambridge University Press, 2002)
The Biggest Bangs: The Mystery of Gamma-Ray Bursts, The Most Violent Explosions in the Universe by Jonathan Katz (Oxford University Press, 2002)
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: [email protected].
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Keith Cooper is a freelance science journalist and editor in the United Kingdom, and has a degree in physics and astrophysics from the University of Manchester. He's the author of "The Contact Paradox: Challenging Our Assumptions in the Search for Extraterrestrial Intelligence" (Bloomsbury Sigma, 2020) and has written articles on astronomy, space, physics and astrobiology for a multitude of magazines and websites.
A 'snowball fight' may help scientists find life on Jupiter's moon Europa
Arrokoth the 'space snowman' and other Kuiper Belt objects may be packed with ancient ice
Cannibal stars at the heart of the Milky Way stay young in a gruesome way
- larbud Interesting; no mention of 1859. Reply
- billslugg The 1859 Carrington event was caused by a stream of charged particles from a solar flare. Any gamma rays emitted would have been blocked by the Earth's atmosphere. Reply
- View All 2 Comments
Most Popular
By Fran Ruiz January 29, 2024
By Fran Ruiz January 26, 2024
By Conor Feehly January 05, 2024
By Keith Cooper December 22, 2023
By Fran Ruiz December 20, 2023
By Fran Ruiz December 19, 2023
By Fran Ruiz December 18, 2023
By Tantse Walter December 18, 2023
By Robert Lea December 05, 2023
By Robert Lea December 04, 2023
By Robert Lea December 01, 2023
- 2 SpaceX moves Super Heavy booster to pad ahead of 4th Starship flight (photos)
- 3 Ambitious new dark matter-hunting experiment delivers 1st results
- 4 Largest 3D map of our universe could hint that dark energy evolves with time
- 5 China putting finishing touches on seaside spaceport for commercial launches (video)

How Light Travels: The Reason Why Telescopes Can See the Invisible Parts of Our Universe
Due to how light travels, we can only see the most eye-popping details of space—like nebulas, supernovas, and black holes—with specialized telescopes.
- Our eyes can see only a tiny fraction of these wavelengths , but our instruments enable us to learn far more.
- Here, we outline how various telescopes detect different wavelengths of light from space.
Light travels only one way: in a straight line. But the path it takes from Point A to Point B is always a waveform, with higher-energy light traveling in shorter wavelengths. Photons , which are tiny parcels of energy, have been traveling across the universe since they first exploded from the Big Bang . They always travel through the vacuum of space at 186,400 miles per second—the speed of light—which is faster than anything else.
Too bad we can glimpse only about 0.0035 percent of the light in the universe with our naked eyes. Humans can perceive just a tiny sliver of the electromagnetic spectrum: wavelengths from about 380–750 nanometers. This is what we call the visible part of the electromagnetic spectrum. The universe may be lovely to look at in this band, but our vision skips right over vast ranges of wavelengths that are either shorter or longer than this limited range. On either side of the visible band lies evidence of interstellar gas clouds, the hottest stars in the universe, gas clouds between galaxies , the gas that rushes into black holes, and much more.
Fortunately, telescopes allow us to see what would otherwise remain hidden. To perceive gas clouds between stars and galaxies, we use detectors that can capture infrared wavelengths. Super-hot stars require instruments that see short, ultraviolet wavelengths. To see the gas clouds between galaxies, we need X-ray detectors.
We’ve been using telescopes designed to reveal the invisible parts of the cosmos for more than 60 years. Because Earth’s atmosphere absorbs most wavelengths of light, many of our telescopes must observe the cosmos from orbit or outer space.
Here’s a snapshot of how we use specialized detectors to explore how light travels across the universe.
Infrared Waves

We can’t see infrared waves, but we can feel them as heat . A sensitive detector like the James Webb Space Telescope can discern this thermal energy from far across the universe. But we use infrared in more down-to-Earth ways as well. For example, remote-control devices work by sending infrared signals at about 940 nanometers to your television or stereo. These heat waves also emanate from incubators to help hatch a chick or keep a pet reptile warm. As a warm being, you radiate infrared waves too; a person using night vision goggles can see you, because the goggles turn infrared energy into false-color optical energy that your eyes can perceive. Infrared telescopes let us see outer space in a similar way.
Astronomers began the first sky surveys with infrared telescopes in the 1960s and 1970s. Webb , launched in 2021, takes advantage of the infrared spectrum to probe the deepest regions of the universe. Orbiting the sun at a truly cold expanse—about one million miles from Earth—Webb has three infrared detectors with the ability to peer farther back in time than any other telescope has so far.
Its primary imaging device, the Near Infrared Camera (NIRCam), observes the universe through detectors tuned to incoming wavelengths ranging from 0.6 to 5 microns, ideal for seeing light from the universe’s earliest stars and galaxies. Webb’s Mid-Infrared Instrument (MIRI) covers the wavelength range from 5 to 28 microns, its sensitive detectors collecting the redshifted light of distant galaxies. Conveniently for us, infrared passes more cleanly through deep space gas and dust clouds, revealing the objects behind them; for this and many other reasons, the infrared spectrum has gained a crucial foothold in our cosmic investigations. Earth-orbiting satellites like NASA’s Wide Field Infrared Survey Telescope ( WFIRST ) observe deep space via longer infrared wavelengths, too.
Yet, when stars first form, they mostly issue ultraviolet light . So why don’t we use ultraviolet detectors to find distant galaxies? It’s because the universe has been stretching since its beginning, and the light that travels through it has been stretching, too; every planet, star, and galaxy continually moves away from everything else. By the time light from GLASS-z13—formed 300 million years after the Big Bang—reaches our telescopes, it has been traveling for more than 13 billion years , a vast distance all the way from a younger universe. The light may have started as ultraviolet waves, but over vast scales of time and space, it ended up as infrared. So, this fledgling galaxy appears as a red dot to NIRCam. We are gazing back in time at a galaxy that is rushing away from us.
Radio Waves
If we could see the night sky only through radio waves, we would notice swaths of supernovae , pulsars, quasars, and gassy star-forming regions instead of the usual pinprick fairy lights of stars and planets.
Tools like the Arecibo Observatory in Puerto Rico can do the job our eyes can’t: detect some of the longest electromagnetic waves in the universe. Radio waves are typically the length of a football field, but they can be even longer than our planet’s diameter. Though the 1,000-foot-wide dish at Arecibo collapsed in 2020 due to structural problems, other large telescopes carry on the work of looking at radio waves from space. Large radio telescopes are special because they actually employ many smaller dishes, integrating their data to produce a really sharp image.
Unlike optical astronomy, ground-based radio telescopes don’t need to contend with clouds and rain. They can make out the composition, structure, and motion of planets and stars no matter the weather. However, the dishes of radio telescopes need to be much larger than optical ones to generate a comparable image, since radio waves are so long. The Parkes Observatory’s dish is 64 meters wide, but its imaging is comparable to a small backyard optical telescope, according to NASA .
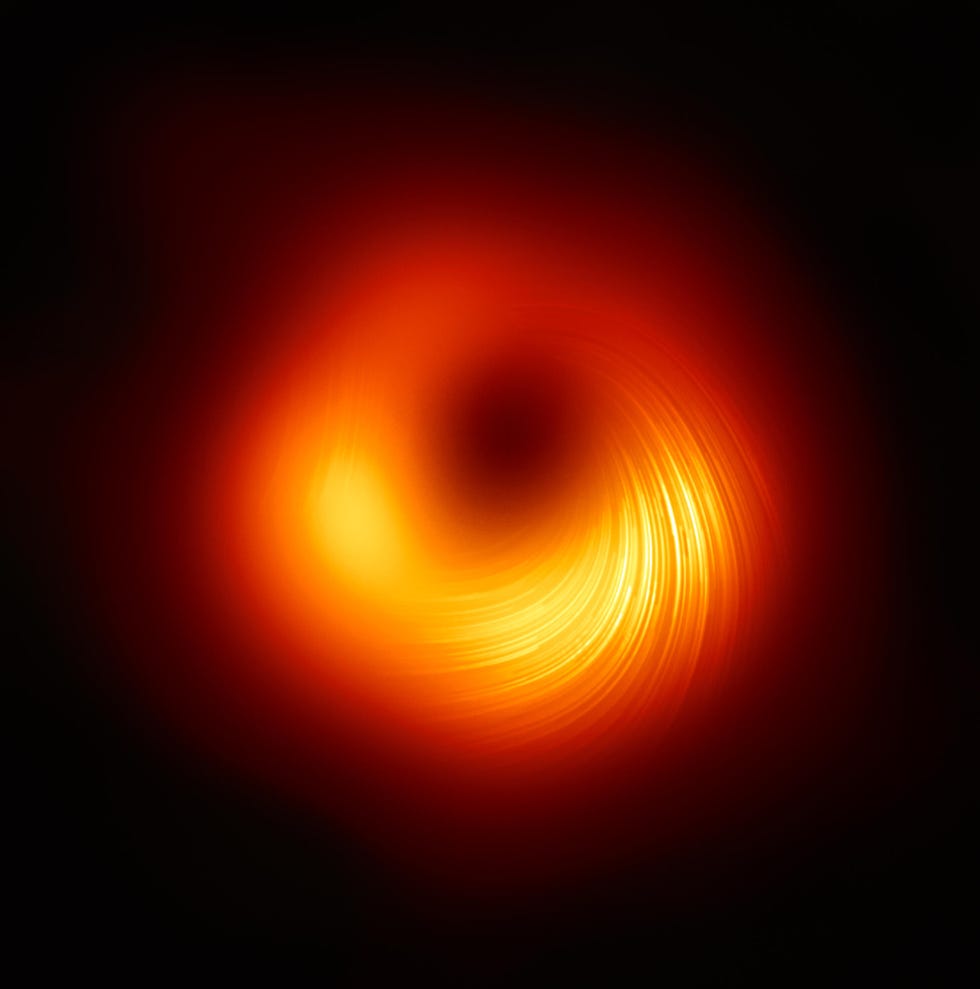
Eight different radio telescopes all over the world coordinated their observations for the Event Horizon Telescope in 2019 to put together the eye-opening image of a black hole in the heart of the M87 galaxy (above).
Ultraviolet Waves
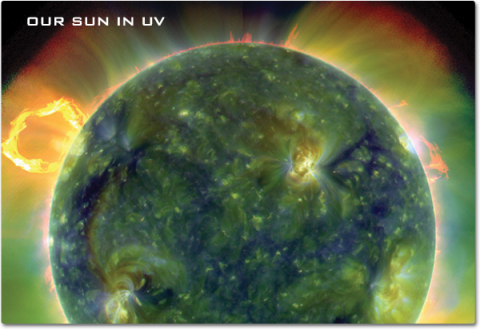
You may be most familiar with ultraviolet, or UV rays, in warnings to use sunscreen . The sun is our greatest local emitter of these higher-frequency, shorter wavelengths just beyond the human visible spectrum, ranging from 100 to 400 nanometers. The Hubble Space Telescope has been our main instrument for observing UV light from space, including young stars forming in Spiral Galaxy NGC 3627, the auroras of Jupiter, and a giant cloud of hydrogen evaporating from an exoplanet that is reacting to its star’s extreme radiation.
Our sun and other stars emit a full range of UV light, telling astronomers how relatively hot or cool they are according to the subdivisions of ultraviolet radiation: near ultraviolet, middle ultraviolet, far ultraviolet, and extreme ultraviolet. Applying a false-color visible light composite lets us see with our own eyes the differences in a star’s gas temperatures.
Hubble’s Wide Field Camera 3 (WFC3) breaks down ultraviolet light into specific present colors with filters. “Science visuals developers assign primary colors and reconstruct the data into a picture our eyes can clearly identify,” according to the Hubble website . Using image-processing software, astronomers and even amateur enthusiasts can turn the UV data into images that are not only beautiful, but also informative.
X-Ray Light
Since 1999, the orbiting Chandra X-Ray Observatory is the most sensitive radio telescope ever built. During one observation that lasted a few hours, its X-ray vision saw only four photons from a galaxy 240 million light-years away, but it was enough to ascertain a novel type of exploding star . The observatory, located 86,500 miles above Earth, can produce detailed, full-color images of hot X-ray-emitting objects, like supernovas, clusters of galaxies and gases, and jets of energy surrounding black holes that are millions of degrees Celsius. It can also measure the intensity of an individual X-ray wavelength, which ranges from just 0.01 to 10 nanometers. Its four sensitive mirrors pick up energetic photons and then electronic detectors at the end of a 30-foot optical apparatus focus the beams of X-rays.
Closer to home, the Aurora Borealis at the poles emits X-rays too. And down on Earth, this high-frequency, low-wavelength light passes easily through the soft tissue of our bodies, but not our bones, yielding stellar X-ray images of our skeletons and teeth.
Visible Light

Visible color gives astronomers essential clues to a whole world of information about a star, including temperature, distance, mass, and chemical composition. The Hubble Telescope, perched 340 miles above our planet, has been a major source of visible light images of the cosmos since 1990.
Hotter objects, like young stars, radiate energy at shorter wavelengths of light; that’s why younger stars at temperatures up to 12,000 degrees Celsius, like the star Rigel, look blue to us. Astronomers can also tell the mass of a star from its color. Because mass corresponds to temperature, observers know that hot blue stars are at least three times the mass of the sun. For instance, the extremely hot, luminous blue variable star Eta Carina’s bulk is 150 times the mass of our sun, and it radiates 1,000,000 times our sun’s energy.
Our comparatively older, dimmer sun is about 5,500 degrees Celsius, so it appears yellow. At the other end of the scale, the old star Betelgeuse has been blowing off its outer layer for the past few years, and it looks red because it’s only about 3,000 degrees Celsius.
A View of Earth
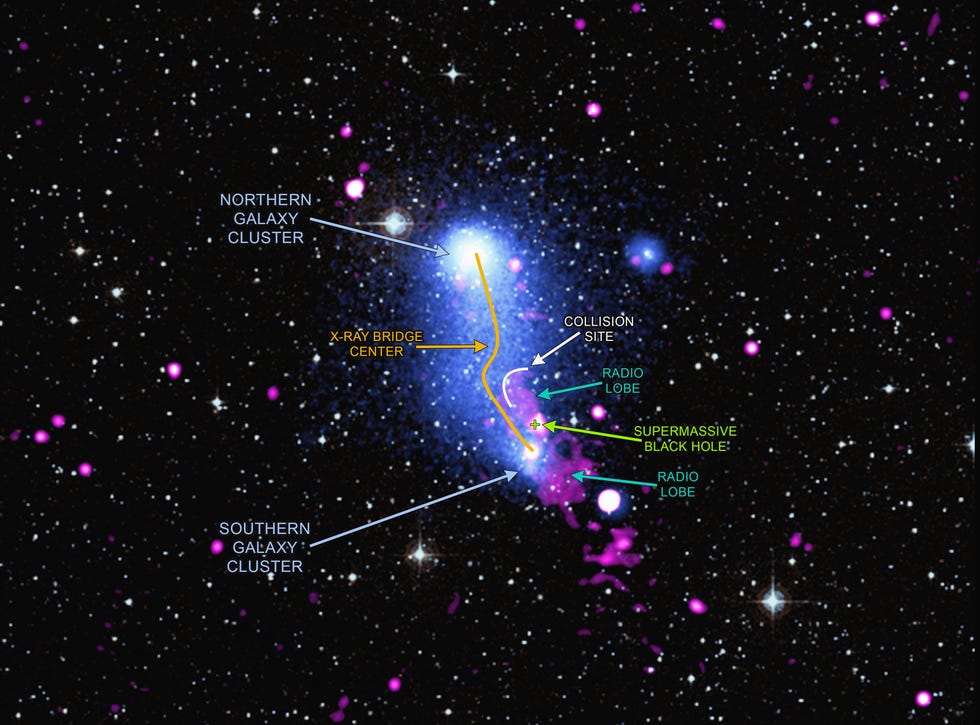
Scientists use different wavelengths of light to study phenomena closer to home, too.
Detectors in orbit can distinguish between geophysical and environmental features on Earth’s changing surface, such as volcanic action. For example, infrared light used alongside visible light detection reveals areas covered in snow, volcanic ash, and vegetation. The Moderate Resolution Imaging Spectroradiometer ( MODIS ) infrared instrument onboard the Aqua and Terra satellites monitors forest fire smoke and locates the source of a fire so humans don’t have to fly through smoke to evaluate the situation.
Next year, a satellite will be launched to gauge forest biomass using a special radar wavelength of about 70 centimeters that can penetrate the leafy canopy.
💡 Why is the sky blue? During the day, oxygen and nitrogen in Earth’s atmosphere scatters electromagnetic energy at the wavelengths of blue light (450–485 nanometers). At sunset, the sun’s light makes a longer journey through the atmosphere before greeting your eyes. Along the way, more of the sun’s light is scattered out of the blue spectrum and deeper into yellow and red.
Before joining Popular Mechanics , Manasee Wagh worked as a newspaper reporter, a science journalist, a tech writer, and a computer engineer. She’s always looking for ways to combine the three greatest joys in her life: science, travel, and food.

.css-cuqpxl:before{padding-right:0.3125rem;content:'//';display:inline;} Pop Mech Pro: Science .css-xtujxj:before{padding-left:0.3125rem;content:'//';display:inline;}
Could the Chair You Sit on Have a Soul?

Here’s How We Could Live in Trees

The Engine Driving Our Oceans Could Die by 2100

Can AI Help Solve Math’s Thorniest Mysteries?

You Can Give Your Body Back to Nature When You Die

How Does UFO Footage Play Tricks on Your Mind?

Why Doesn’t the Living Human Body ‘Go Bad’?
Is the Room-Temperature Superconductor Back?

What 9 Months on a Cruise Ship Can Do to You

Scientists Just Figured Out Why Pee is Yellow

5 Alien Hoaxes That Prove We Truly Want to Believe
16.5 The Electromagnetic Spectrum
Learning objectives.
By the end of this section, you will be able to:
- Explain how electromagnetic waves are divided into different ranges, depending on wavelength and corresponding frequency
- Describe how electromagnetic waves in different categories are produced
- Describe some of the many practical everyday applications of electromagnetic waves
Electromagnetic waves have a vast range of practical everyday applications that includes such diverse uses as communication by cell phone and radio broadcasting, WiFi, cooking, vision, medical imaging, and treating cancer. In this module, we discuss how electromagnetic waves are classified into categories such as radio, infrared, ultraviolet, and so on. We also summarize some of the main applications for each range.
The different categories of electromagnetic waves differ in their wavelength range, or equivalently, in their corresponding frequency ranges. Their properties change smoothly from one frequency range to the next, with different applications in each range. A brief overview of the production and utilization of electromagnetic waves is found in Table 16.1 .
The relationship c = f λ c = f λ between frequency f and wavelength λ λ applies to all waves and ensures that greater frequency means smaller wavelength. Figure 16.17 shows how the various types of electromagnetic waves are categorized according to their wavelengths and frequencies—that is, it shows the electromagnetic spectrum.
Radio Waves
The term radio waves refers to electromagnetic radiation with wavelengths greater than about 0.1 m. Radio waves are commonly used for audio communications (i.e., for radios), but the term is used for electromagnetic waves in this range regardless of their application. Radio waves typically result from an alternating current in the wires of a broadcast antenna. They cover a very broad wavelength range and are divided into many subranges, including microwaves, electromagnetic waves used for AM and FM radio, cellular telephones, and TV signals.
There is no lowest frequency of radio waves, but ELF waves, or “extremely low frequency” are among the lowest frequencies commonly encountered, from 3 Hz to 3 kHz. The accelerating charge in the ac currents of electrical power lines produce electromagnetic waves in this range. ELF waves are able to penetrate sea water, which strongly absorbs electromagnetic waves of higher frequency, and therefore are useful for submarine communications.
In order to use an electromagnetic wave to transmit information, the amplitude, frequency, or phase of the wave is modulated , or varied in a controlled way that encodes the intended information into the wave. In AM radio transmission, the amplitude of the wave is modulated to mimic the vibrations of the sound being conveyed. Fourier’s theorem implies that the modulated AM wave amounts to a superposition of waves covering some narrow frequency range. Each AM station is assigned a specific carrier frequency that, by international agreement, is allowed to vary by ±5 kHz ±5 kHz . In FM radio transmission, the frequency of the wave is modulated to carry this information, as illustrated in Figure 16.18 , and the frequency of each station is allowed to use 100 kHz on each side of its carrier frequency. The electromagnetic wave produces a current in a receiving antenna, and the radio or television processes the signal to produce the sound and any image. The higher the frequency of the radio wave used to carry the data, the greater the detailed variation of the wave that can be carried by modulating it over each time unit, and the more data that can be transmitted per unit of time. The assigned frequencies for AM broadcasting are 540 to 1600 kHz, and for FM are 88 MHz to108 MHz.
Cell phone conversations, and television voice and video images are commonly transmitted as digital data, by converting the signal into a sequence of binary ones and zeros. This allows clearer data transmission when the signal is weak, and allows using computer algorithms to compress the digital data to transmit more data in each frequency range. Computer data as well is transmitted as a sequence of binary ones and zeros, each one or zero constituting one bit of data.
Microwaves are the highest-frequency electromagnetic waves that can be produced by currents in macroscopic circuits and devices. Microwave frequencies range from about 10 9 Hz 10 9 Hz to nearly 10 12 Hz 10 12 Hz . Their high frequencies correspond to short wavelengths compared with other radio waves—hence the name “microwave.” Microwaves also occur naturally as the cosmic background radiation left over from the origin of the universe. Along with other ranges of electromagnetic waves, they are part of the radiation that any object above absolute zero emits and absorbs because of thermal agitation , that is, from the thermal motion of its atoms and molecules.
Most satellite-transmitted information is carried on microwaves . Radar is a common application of microwaves. By detecting and timing microwave echoes, radar systems can determine the distance to objects as diverse as clouds, aircraft, or even the surface of Venus.
Microwaves of 2.45 GHz are commonly used in microwave ovens. The electrons in a water molecule tend to remain closer to the oxygen nucleus than the hydrogen nuclei ( Figure 16.19 ). This creates two separated centers of equal and opposite charges, giving the molecule a dipole moment (see Electric Field ). The oscillating electric field of the microwaves inside the oven exerts a torque that tends to align each molecule first in one direction and then in the other, with the motion of each molecule coupled to others around it. This pumps energy into the continual thermal motion of the water to heat the food. The plate under the food contains no water, and remains relatively unheated.
The microwaves in a microwave oven reflect off the walls of the oven, so that the superposition of waves produces standing waves, similar to the standing waves of a vibrating guitar or violin string (see Normal Modes of a Standing Sound Wave ). A rotating fan acts as a stirrer by reflecting the microwaves in different directions, and food turntables, help spread out the hot spots.
Example 16.8
Why microwave ovens heat unevenly, significance.
A cell phone has a radio receiver and a weak radio transmitter, both of which can quickly tune to hundreds of specifically assigned microwave frequencies. The low intensity of the transmitted signal gives it an intentionally limited range. A ground-based system links the phone to only to the broadcast tower assigned to the specific small area, or cell, and smoothly transitions its connection to the next cell when the signal reception there is the stronger one. This enables a cell phone to be used while changing location.
Microwaves also provide the WiFi that enables owners of cell phones, laptop computers, and similar devices to connect wirelessly to the Internet at home and at coffee shops and airports. A wireless WiFi router is a device that exchanges data over the Internet through the cable or another connection, and uses microwaves to exchange the data wirelessly with devices such as cell phones and computers. The term WiFi itself refers to the standards followed in modulating and analyzing the microwaves so that wireless routers and devices from different manufacturers work compatibly with one another. The computer data in each direction consist of sequences of binary zeros and ones, each corresponding to a binary bit. The microwaves are in the range of 2.4 GHz to 5.0 GHz range.
Other wireless technologies also use microwaves to provide everyday communications between devices. Bluetooth developed alongside WiFi as a standard for radio communication in the 2.4-GHz range between nearby devices, for example, to link to headphones and audio earpieces to devices such as radios, or a driver’s cell phone to a hands-free device to allow answering phone calls without fumbling directly with the cell phone.
Microwaves find use also in radio tagging, using RFID (radio frequency identification) technology. Examples are RFID tags attached to store merchandize, transponder for toll booths use attached to the windshield of a car, or even a chip embedded into a pet’s skin. The device responds to a microwave signal by emitting a signal of its own with encoded information, allowing stores to quickly ring up items at their cash registers, drivers to charge tolls to their account without stopping, and lost pets to be reunited with their owners. NFC (near field communication) works similarly, except it is much shorter range. Its mechanism of interaction is the induced magnetic field at microwave frequencies between two coils. Cell phones that have NFC capability and the right software can supply information for purchases using the cell phone instead of a physical credit card. The very short range of the data transfer is a desired security feature in this case.
Infrared Radiation
The boundary between the microwave and infrared regions of the electromagnetic spectrum is not well defined (see Figure 16.17 ). Infrared radiation is generally produced by thermal motion, and the vibration and rotation of atoms and molecules. Electronic transitions in atoms and molecules can also produce infrared radiation . About half of the solar energy arriving at Earth is in the infrared region, with most of the rest in the visible part of the spectrum. About 23% of the solar energy is absorbed in the atmosphere, about 48% is absorbed at Earth’s surface, and about 29% is reflected back into space. 1
The range of infrared frequencies extends up to the lower limit of visible light, just below red. In fact, infrared means “below red.” Water molecules rotate and vibrate particularly well at infrared frequencies. Reconnaissance satellites can detect buildings, vehicles, and even individual humans by their infrared emissions, whose power radiation is proportional to the fourth power of the absolute temperature. More mundanely, we use infrared lamps, including those called quartz heaters , to preferentially warm us because we absorb infrared better than our surroundings.
The familiar handheld “remotes” for changing channels and settings on television sets often transmit their signal by modulating an infrared beam. If you try to use a TV remote without the infrared emitter being in direct line of sight with the infrared detector, you may find the television not responding. Some remotes use Bluetooth instead and reduce this annoyance.
Visible Light
Visible light is the narrow segment of the electromagnetic spectrum between about 400 nm and about 750 nm to which the normal human eye responds. Visible light is produced by vibrations and rotations of atoms and molecules, as well as by electronic transitions within atoms and molecules. The receivers or detectors of light largely utilize electronic transitions.
Red light has the lowest frequencies and longest wavelengths, whereas violet has the highest frequencies and shortest wavelengths ( Figure 16.20 ). Blackbody radiation from the Sun peaks in the visible part of the spectrum but is more intense in the red than in the violet, making the sun yellowish in appearance.
Living things—plants and animals—have evolved to utilize and respond to parts of the electromagnetic spectrum in which they are embedded. We enjoy the beauty of nature through visible light. Plants are more selective. Photosynthesis uses parts of the visible spectrum to make sugars.
Ultraviolet Radiation
Ultraviolet means “above violet.” The electromagnetic frequencies of ultraviolet radiation (UV) extend upward from violet, the highest-frequency visible light. The highest-frequency ultraviolet overlaps with the lowest-frequency X-rays. The wavelengths of ultraviolet extend from 400 nm down to about 10 nm at its highest frequencies. Ultraviolet is produced by atomic and molecular motions and electronic transitions.
UV radiation from the Sun is broadly subdivided into three wavelength ranges: UV-A (320–400 nm) is the lowest frequency, then UV-B (290–320 nm) and UV-C (220–290 nm). Most UV-B and all UV-C are absorbed by ozone ( O 3 O 3 ) molecules in the upper atmosphere. Consequently, 99% of the solar UV radiation reaching Earth’s surface is UV-A.
Sunburn is caused by large exposures to UV-B and UV-C, and repeated exposure can increase the likelihood of skin cancer. The tanning response is a defense mechanism in which the body produces pigments in inert skin layers to reduce exposure of the living cells below. However, tanning is not sufficient protection, and people are generally advised to avoid prolonged exposure to sunlight and to wear sunscreen or protective clothing when outside — even on cloudy days.
Because it absorbs harmful UV radiation, ozone is important to many life forms and to the overall climate. Ozone is depleted by chemicals, which can result in areas of lower concentration -- often referred to as "holes" although the term is entirely accurate. Mario Molina and F. Sherwood Rowland found that chlorofluorocarbons (CFCs), which were extremely common commercial substances, were responsible for ozone depletion. UV radiation itself broke down CFCs, releasing chlorine atoms that reacted with and eliminated ozone. Other substances have since been proven to have the same effect.
As examined in a later chapter, the shorter the wavelength of light, the greater the energy change of an atom or molecule that absorbs the light in an electronic transition. This makes short-wavelength ultraviolet light damaging to living cells. It also explains why ultraviolet radiation is better able than visible light to cause some materials to glow, or fluoresce .
Besides the adverse effects of ultraviolet radiation, there are also benefits of exposure in nature and uses in technology. Vitamin D production in the skin results from exposure to UV-B radiation, generally from sunlight. Several studies suggest vitamin D deficiency is associated with the development of a range of cancers (prostate, breast, colon), as well as osteoporosis. Low-intensity ultraviolet has applications such as providing the energy to cause certain dyes to fluoresce and emit visible light, for example, in printed money to display hidden watermarks as counterfeit protection.
One of the first illustrations of UV light’s impact on Earth occurred during the Apollo 16 mission in 1972. The mission included the first astronomical images taken from the moon, using a compact and resilient Far Ultraviolet Camera/Spectrograph designed for moon use by scientist and inventor George Robert Carruthers. Designed to capture UV images without the obscuring effects of the Earth’s atmosphere, its most famous image was of the planet itself. Carruthers, who also trained the astronauts on the device’s use, mentioned afterward that “the most immediately obvious and spectacular results were really for the Earth observations, because this was the first time that the Earth had been photographed from a distance in ultraviolet (UV) light, so that you could see the full extent of the hydrogen atmosphere, the polar auroris and what we call the tropical airglow belt.”
X-ray s have wavelengths from about 10 − 8 m to 10 − 12 m 10 − 8 m to 10 − 12 m . They have shorter wavelengths, and higher frequencies, than ultraviolet, so that the energy they transfer at an atomic level is greater. As a result, X-rays have adverse effects on living cells similar to those of ultraviolet radiation, but they are more penetrating. Cancer and genetic defects can be induced by X-rays. Because of their effect on rapidly dividing cells, X-rays can also be used to treat and even cure cancer.
The widest use of X-rays is for imaging objects that are opaque to visible light, such as the human body or aircraft parts. In humans, the risk of cell damage is weighed carefully against the benefit of the diagnostic information obtained.
Soon after nuclear radioactivity was first detected in 1896, it was found that at least three distinct types of radiation were being emitted, and these were designated as alpha, beta, and gamma rays. The most penetrating nuclear radiation, the gamma ray ( γ γ ray) , was later found to be an extremely high-frequency electromagnetic wave.
The lower end of the γ - γ - ray frequency range overlaps the upper end of the X-ray range. Gamma rays have characteristics identical to X-rays of the same frequency—they differ only in source. The name “gamma rays” is generally used for electromagnetic radiation emitted by a nucleus, while X-rays are generally produced by bombarding a target with energetic electrons in an X-ray tube. At higher frequencies, γ γ rays are more penetrating and more damaging to living tissue. They have many of the same uses as X-rays, including cancer therapy. Gamma radiation from radioactive materials is used in nuclear medicine.
Interactive
Engage the Phet simulation below to explore how light interacts with molecules in our atmosphere. Notice that the absorption of light depends on the molecule and the type of light. Then, relate the energy of the light to the resulting motion. Notice that energy increases from microwave to ultraviolet. Lastly, predict the motion of a molecule based on the type of light it absorbs.
Check Your Understanding 16.6
How do the electromagnetic waves for the different kinds of electromagnetic radiation differ?
- 1 http://earthobservatory.nasa.gov/Features/EnergyBalance/page4.php
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/university-physics-volume-2/pages/1-introduction
- Authors: Samuel J. Ling, William Moebs, Jeff Sanny
- Publisher/website: OpenStax
- Book title: University Physics Volume 2
- Publication date: Oct 6, 2016
- Location: Houston, Texas
- Book URL: https://openstax.org/books/university-physics-volume-2/pages/1-introduction
- Section URL: https://openstax.org/books/university-physics-volume-2/pages/16-5-the-electromagnetic-spectrum
© Jan 19, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Radiation and Spectra
The electromagnetic spectrum, learning objectives.
By the end of this section, you will be able to:
- Understand the bands of the electromagnetic spectrum and how they differ from one another
- Understand how each part of the spectrum interacts with Earth’s atmosphere
- Explain how and why the light emitted by an object depends on its temperature
Objects in the universe send out an enormous range of electromagnetic radiation. Scientists call this range the electromagnetic spectrum , which they have divided into a number of categories. The spectrum is shown in [link] , with some information about the waves in each part or band.

Types of Electromagnetic Radiation
Electromagnetic radiation with the shortest wavelengths, no longer than 0.01 nanometer, is categorized as gamma rays (1 nanometer = 10 –9 meters; see Appendix D ). The name gamma comes from the third letter of the Greek alphabet: gamma rays were the third kind of radiation discovered coming from radioactive atoms when physicists first investigated their behavior. Because gamma rays carry a lot of energy, they can be dangerous for living tissues. Gamma radiation is generated deep in the interior of stars, as well as by some of the most violent phenomena in the universe, such as the deaths of stars and the merging of stellar corpses. Gamma rays coming to Earth are absorbed by our atmosphere before they reach the ground (which is a good thing for our health); thus, they can only be studied using instruments in space.
Electromagnetic radiation with wavelengths between 0.01 nanometer and 20 nanometers is referred to as X-rays . Being more energetic than visible light, X-rays are able to penetrate soft tissues but not bones, and so allow us to make images of the shadows of the bones inside us. While X-rays can penetrate a short length of human flesh, they are stopped by the large numbers of atoms in Earth’s atmosphere with which they interact. Thus, X-ray astronomy (like gamma-ray astronomy) could not develop until we invented ways of sending instruments above our atmosphere ( [link] ).

Radiation intermediate between X-rays and visible light is ultraviolet (meaning higher energy than violet). Outside the world of science, ultraviolet light is sometimes called “black light” because our eyes cannot see it. Ultraviolet radiation is mostly blocked by the ozone layer of Earth’s atmosphere, but a small fraction of ultraviolet rays from our Sun do penetrate to cause sunburn or, in extreme cases of overexposure, skin cancer in human beings. Ultraviolet astronomy is also best done from space.
Electromagnetic radiation with wavelengths between roughly 400 and 700 nm is called visible light because these are the waves that human vision can perceive. This is also the band of the electromagnetic spectrum that most readily reaches Earth’s surface. These two observations are not coincidental: human eyes evolved to see the kinds of waves that arrive from the Sun most effectively. Visible light penetrates Earth’s atmosphere effectively, except when it is temporarily blocked by clouds.
Between visible light and radio waves are the wavelengths of infrared or heat radiation. Astronomer William Herschel first discovered infrared in 1800 while trying to measure the temperatures of different colors of sunlight spread out into a spectrum. He noticed that when he accidently positioned his thermometer beyond the reddest color, it still registered heating due to some invisible energy coming from the Sun. This was the first hint about the existence of the other (invisible) bands of the electromagnetic spectrum, although it would take many decades for our full understanding to develop.
A heat lamp radiates mostly infrared radiation, and the nerve endings in our skin are sensitive to this band of the electromagnetic spectrum. Infrared waves are absorbed by water and carbon dioxide molecules, which are more concentrated low in Earth’s atmosphere. For this reason, infrared astronomy is best done from high mountaintops, high-flying airplanes, and spacecraft.
After infrared comes the familiar microwave , used in short-wave communication and microwave ovens. (Wavelengths vary from 1 millimeter to 1 meter and are absorbed by water vapor, which makes them effective in heating foods.) The “micro-” prefix refers to the fact that microwaves are small in comparison to radio waves, the next on the spectrum. You may remember that tea—which is full of water—heats up quickly in your microwave oven, while a ceramic cup—from which water has been removed by baking—stays cool in comparison.
All electromagnetic waves longer than microwaves are called radio waves , but this is so broad a category that we generally divide it into several subsections. Among the most familiar of these are radar waves, which are used in radar guns by traffic officers to determine vehicle speeds, and AM radio waves, which were the first to be developed for broadcasting. The wavelengths of these different categories range from over a meter to hundreds of meters, and other radio radiation can have wavelengths as long as several kilometers.
With such a wide range of wavelengths, not all radio waves interact with Earth’s atmosphere in the same way. FM and TV waves are not absorbed and can travel easily through our atmosphere. AM radio waves are absorbed or reflected by a layer in Earth’s atmosphere called the ionosphere (the ionosphere is a layer of charged particles at the top of our atmosphere, produced by interactions with sunlight and charged particles that are ejected from the Sun).
We hope this brief survey has left you with one strong impression: although visible light is what most people associate with astronomy, the light that our eyes can see is only a tiny fraction of the broad range of waves generated in the universe. Today, we understand that judging some astronomical phenomenon by using only the light we can see is like hiding under the table at a big dinner party and judging all the guests by nothing but their shoes. There’s a lot more to each person than meets our eye under the table. It is very important for those who study astronomy today to avoid being “visible light chauvinists”—to respect only the information seen by their eyes while ignoring the information gathered by instruments sensitive to other bands of the electromagnetic spectrum.
[link] summarizes the bands of the electromagnetic spectrum and indicates the temperatures and typical astronomical objects that emit each kind of electromagnetic radiation. While at first, some of the types of radiation listed in the table may seem unfamiliar, you will get to know them better as your astronomy course continues. You can return to this table as you learn more about the types of objects astronomers study.
Radiation and Temperature
Some astronomical objects emit mostly infrared radiation, others mostly visible light, and still others mostly ultraviolet radiation. What determines the type of electromagnetic radiation emitted by the Sun, stars, and other dense astronomical objects? The answer often turns out to be their temperature .
At the microscopic level, everything in nature is in motion. A solid is composed of molecules and atoms in continuous vibration: they move back and forth in place, but their motion is much too small for our eyes to make out. A gas consists of atoms and/or molecules that are flying about freely at high speed, continually bumping into one another and bombarding the surrounding matter. The hotter the solid or gas, the more rapid the motion of its molecules or atoms. The temperature of something is thus a measure of the average motion energy of the particles that make it up.
This motion at the microscopic level is responsible for much of the electromagnetic radiation on Earth and in the universe. As atoms and molecules move about and collide, or vibrate in place, their electrons give off electromagnetic radiation. The characteristics of this radiation are determined by the temperature of those atoms and molecules. In a hot material, for example, the individual particles vibrate in place or move rapidly from collisions, so the emitted waves are, on average, more energetic. And recall that higher energy waves have a higher frequency. In very cool material, the particles have low-energy atomic and molecular motions and thus generate lower-energy waves.
Check out the NASA briefing or NASA’s 5-minute introductory video to learn more about the electromagnetic spectrum.
Radiation Laws
To understand, in more quantitative detail, the relationship between temperature and electromagnetic radiation, we imagine an idealized object called a blackbody . Such an object (unlike your sweater or your astronomy instructor’s head) does not reflect or scatter any radiation, but absorbs all the electromagnetic energy that falls onto it. The energy that is absorbed causes the atoms and molecules in it to vibrate or move around at increasing speeds. As it gets hotter, this object will radiate electromagnetic waves until absorption and radiation are in balance. We want to discuss such an idealized object because, as you will see, stars behave in very nearly the same way.
The radiation from a blackbody has several characteristics, as illustrated in [link] . The graph shows the power emitted at each wavelength by objects of different temperatures. In science, the word power means the energy coming off per second (and it is typically measured in watts , which you are probably familiar with from buying lightbulbs).

First of all, notice that the curves show that, at each temperature, our blackbody object emits radiation (photons) at all wavelengths (all colors). This is because in any solid or denser gas, some molecules or atoms vibrate or move between collisions slower than average and some move faster than average. So when we look at the electromagnetic waves emitted, we find a broad range, or spectrum, of energies and wavelengths. More energy is emitted at the average vibration or motion rate (the highest part of each curve), but if we have a large number of atoms or molecules, some energy will be detected at each wavelength.
Second, note that an object at a higher temperature emits more power at all wavelengths than does a cooler one. In a hot gas (the taller curves in [link] ), for example, the atoms have more collisions and give off more energy. In the real world of stars, this means that hotter stars give off more energy at every wavelength than do cooler stars.
Third, the graph shows us that the higher the temperature, the shorter the wavelength at which the maximum power is emitted. Remember that a shorter wavelength means a higher frequency and energy. It makes sense, then, that hot objects give off a larger fraction of their energy at shorter wavelengths (higher energies) than do cool objects. You may have observed examples of this rule in everyday life. When a burner on an electric stove is turned on low, it emits only heat, which is infrared radiation, but does not glow with visible light. If the burner is set to a higher temperature, it starts to glow a dull red. At a still-higher setting, it glows a brighter orange-red (shorter wavelength). At even higher temperatures, which cannot be reached with ordinary stoves, metal can appear brilliant yellow or even blue-white.
We can use these ideas to come up with a rough sort of “thermometer” for measuring the temperatures of stars. Because many stars give off most of their energy in visible light, the color of light that dominates a star’s appearance is a rough indicator of its temperature. If one star looks red and another looks blue, which one has the higher temperature? Because blue is the shorter-wavelength color, it is the sign of a hotter star. (Note that the temperatures we associate with different colors in science are not the same as the ones artists use. In art, red is often called a “hot” color and blue a “cool” color. Likewise, we commonly see red on faucet or air conditioning controls to indicate hot temperatures and blue to indicate cold temperatures. Although these are common uses to us in daily life, in nature, it’s the other way around.)
We can develop a more precise star thermometer by measuring how much energy a star gives off at each wavelength and by constructing diagrams like [link] . The location of the peak (or maximum) in the power curve of each star can tell us its temperature. The average temperature at the surface of the Sun, which is where the radiation that we see is emitted, turns out to be 5800 K. (Throughout this text, we use the kelvin or absolute temperature scale. On this scale, water freezes at 273 K and boils at 373 K. All molecular motion ceases at 0 K. The various temperature scales are described in Appendix D .) There are stars cooler than the Sun and stars hotter than the Sun.
The wavelength at which maximum power is emitted can be calculated according to the equation
where the wavelength is in nanometers (one billionth of a meter) and the temperature is in K. This relationship is called Wien’s law . For the Sun, the wavelength at which the maximum energy is emitted is 520 nanometers, which is near the middle of that portion of the electromagnetic spectrum called visible light. Characteristic temperatures of other astronomical objects, and the wavelengths at which they emit most of their power, are listed in [link] .
Calculating the Temperature of a Blackbody We can use Wien’s law to calculate the temperature of a star provided we know the wavelength of peak intensity for its spectrum. If the emitted radiation from a red dwarf star has a wavelength of maximum power at 1200 nm, what is the temperature of this star, assuming it is a blackbody?
Solution Solving Wien’s law for temperature gives:
Check Your Learning What is the temperature of a star whose maximum light is emitted at a much shorter wavelength of 290 nm?
[latex]T=\frac{3\times {10}^{6}\text{nm K}}{{\text{\lambda }}_{\text{max}}}=\frac{3\times {10}^{6}\text{nm K}}{290\text{nm}}=10,300\text{K}[/latex]
Since this star has a peak wavelength that is at a shorter wavelength (in the ultraviolet part of the spectrum) than that of our Sun (in the visible part of the spectrum), it should come as no surprise that its surface temperature is much hotter than our Sun’s.
We can also describe our observation that hotter objects radiate more power at all wavelengths in a mathematical form. If we sum up the contributions from all parts of the electromagnetic spectrum, we obtain the total energy emitted by a blackbody. What we usually measure from a large object like a star is the energy flux , the power emitted per square meter. The word flux means “flow” here: we are interested in the flow of power into an area (like the area of a telescope mirror). It turns out that the energy flux from a blackbody at temperature T is proportional to the fourth power of its absolute temperature. This relationship is known as the Stefan-Boltzmann law and can be written in the form of an equation as
where F stands for the energy flux and σ (Greek letter sigma) is a constant number (5.67 × 10 8 ).
Notice how impressive this result is. Increasing the temperature of a star would have a tremendous effect on the power it radiates. If the Sun, for example, were twice as hot—that is, if it had a temperature of 11,600 K—it would radiate 2 4 , or 16 times more power than it does now. Tripling the temperature would raise the power output 81 times. Hot stars really shine away a tremendous amount of energy.
Calculating the Power of a Star While energy flux tells us how much power a star emits per square meter, we would often like to know how much total power is emitted by the star. We can determine that by multiplying the energy flux by the number of square meters on the surface of the star. Stars are mostly spherical, so we can use the formula 4π R 2 for the surface area, where R is the radius of the star. The total power emitted by the star (which we call the star’s “absolute luminosity”) can be found by multiplying the formula for energy flux and the formula for the surface area:
Two stars have the same size and are the same distance from us. Star A has a surface temperature of 6000 K, and star B has a surface temperature twice as high, 12,000 K. How much more luminous is star B compared to star A?
Take the ratio of the luminosity of Star A to Star B:
Because the two stars are the same size, R A = R B , leaving
Check Your Learning Two stars with identical diameters are the same distance away. One has a temperature of 8700 K and the other has a temperature of 2900 K. Which is brighter? How much brighter is it?
The 5800 K star has triple the temperature, so it is 3 4 = 81 times brighter.
Key Concepts and Summary
The electromagnetic spectrum consists of gamma rays, X-rays, ultraviolet radiation, visible light, infrared, and radio radiation. Many of these wavelengths cannot penetrate the layers of Earth’s atmosphere and must be observed from space, whereas others—such as visible light, FM radio and TV—can penetrate to Earth’s surface. The emission of electromagnetic radiation is intimately connected to the temperature of the source. The higher the temperature of an idealized emitter of electromagnetic radiation, the shorter is the wavelength at which the maximum amount of radiation is emitted. The mathematical equation describing this relationship is known as Wien’s law: λ max = (3 × 10 6 )/ T . The total power emitted per square meter increases with increasing temperature. The relationship between emitted energy flux and temperature is known as the Stefan-Boltzmann law: F = σ T 4 .
- Astronomy. Provided by : OpenStax CNX. Located at : http://cnx.org/contents/[email protected] . License : CC BY: Attribution . License Terms : Download for free at http://cnx.org/contents/[email protected].
- CTAO Secondments
- How CTAO Works
- Funding Sources
Gamma Rays & Cosmic Sources
- Study Themes
- Key Targets
- CTAO Performance
- CTAO Science Symposium
- CTAO School
- Webinars for Researchers
- Construction Project Overview
- Procurement
- Media Usage
- CTAO Newsletter
- CTAO para Educadores
- Diccionario
- Outreach Webinars
- Building from Diversity
Observing the highest-energy processes in the Universe
Electromagnetic spectrum, to understand gamma rays, it’s important to understand the electromagnetic spectrum. the light we see is actually just a small portion of the total amount of light that surrounds us. the electromagnetic spectrum is used to describe the entire range of light that exists..

Light propagates as waves of alternating electric and magnetic fields that can be measured by its energy, its frequency (number of waves that pass by a point in one second) or its wavelength (the distance from the peak of one wave to the next). The larger the energy, the larger the frequency and the smaller the wavelength, and vice versa. T he electromagnetic spectrum is the classification of light according to these properties . It ranges from the very lowest frequencies or energies of radio waves and microwaves; to the mid-range energies found in infrared, optical (visible) and ultraviolet light; to the very highest frequencies of X-rays and gamma rays. The energy range of gamma rays is so vast that it doesn’ t even have a well-defined upper limit. The gamma rays CTA O will detect are about 10 trillion times more energetic than visible light.
The electromagnetic spectrum provides scientists with a variety of ways to view the Universe. As seen in the figures below, telescopes detecting different frequencies of light provide different perspectives of the cosmic sources, such as the Milky Way and the Crab Nebula, providing a more complete picture of the phenomena they are studying. With its ability to view the highest-energy processes in the Universe, the CTA O will be a vital asset in improving our understanding of these phenomena.

For example, supernova remnants – the giant explosion shells generated by dying stars – are suspected as accelerators of cosmic rays. The CTAO will have the resolution to identify specific regions of supernova remnants and probe the presence of high-energy cosmic rays, that serve as sources of gamma rays.
Top Background Image Credit: ESA/NASA/Felix Mirabel
Non-Thermal Emissions
Most of the light we are used to seeing is emitted by hot objects and is known as thermal radiation. The hotter the source of this radiation, the higher the energy or frequency of the light produced. However, it is not possible for objects to get hot enough to produce gamma rays; these must be produced by a non-thermal mechanism. The mechanisms often rely on the presence of high-energy sub-atomic particles that are produced by some kind of cosmic particle accelerator.
Accelerated particles develop in special environments where a small fraction of the particles can take on an “un-fair” share (or fraction) of the energy available. In such a system, a small number of particles can be accelerated to very close to the speed of light and carry a significant fraction of the energy available. Such energetic particles can then interact with the surrounding magnetic fields , matter or even with light, giving rise to high-energy electromagnetic radiation, such as gamma rays. Since the radiati on emitted is not related to the temperature of the s ource, it is known as non-thermal emission.
These special environments are usually associated with violent events such as explosions, outbursts or powerful jets of material produced close to the giant black holes at the centre of galaxies. For this reason, gamma rays can be used to trace violent events in the U niverse.


Cosmic Sources
The CTA O will be sensitive to the highest-energy gamma rays, making it possible to probe the physical processes at work in some of the most violent environments in the Universe. Although cosmic gamma rays cannot reach the E arth’s surface, the CTA O can detect them from the ground using the subatomic particle cascades that they produce in the atmosphere. Charged particles in these cascades travel faster than the speed of light in the air and emit visible (mostly blue) light known as Cherenkov light. The CTA O ’s large telescope mirrors and ultra-high-speed cameras can then collect and record the nanosecond flash of Cherenkov light so that the incoming gamma ray can be tracked back to its cosmic source. (See How CTAO Works )
The CTA O will be able to detect hundreds of objects in our G alaxy, the Milky Way. These galactic sources will include the remnants of supernova explosions, the rapidly spinning ultra-dense stars known as pulsars and more normal stars in binary systems and large clusters. Beyond the Milky Way, the CTA O will detect star-forming galaxies and galaxies with supermassive black holes at their luminous centres (active galactic nuclei or AGN) and, possibly, whole clusters of galaxies. The gamma rays detected with the CTA O may also provide a direct signature of dark matter, evidence for deviations from Einstein’s theory of relativity and definitive answers to the contents of cosmic voids, the empty space that exists between galaxy filaments in the Universe.
Learn more about the types of cosmic sources the CTA O will be seeking to detect:
Cosmic Rays
Even though they are called “rays,” cosmic rays are really just standard atomic particles. Despite being “normal” matter, cosmic rays are special because they are accelerated to extraordinarily high energies, traveling very close to the speed of light. Primarily in the form of high-energy protons and atomic nuclei, cosmic rays constantly bombard the Earth, but despite a century-long search, we know very little about their origin sources and the role they play in our own Galaxy and beyond. Gamma rays are produced in the interactions of cosmic rays and provide the most sensitive means to study cosmic rays in and around their sources.

Black Holes
Black holes are among the most mysterious objects in astronomy. They are thought to be very small regions in space-time with a gravitational pull so strong that nothing, not even light, can escape. But don’t be fooled by their hallmark “black” – their surroundings are are some of the brightest sources of very-high energy (VHE) gamma rays.
It is believed that most black holes are the relics of massive stars following a supernova explosion. The core of the star collapses under its own gravity to form a black hole, which are typically only a few kilometers in radius but with a mass several times greater than the Sun. When black holes accrete (grow by gravitationally attracting more matter) material from their surroundings, it is a violent, highly energetic process. Much of the material is devoured by the black hole and it grows in size, and the frictional forces within the material spiraling into the black hole make the object immensely luminous.
On a very different size-scale, supermassive black holes are a million to a billion times more massive than the Sun and are assumed to exist in the centre of most galaxies, including our own. While the central black hole in the Milky Way is only detectable through the orbits of stars moving around it, about 10 percent of known galaxies, so-called “active galaxies,” harbour a supermassive black hole that is fueled by a huge accretion disk (a rotating disk of material or gas formed by the black hole’s accretion). The very hot disk can outshine all the stars in the galaxy itself and can produce powerful outflows called “jets,” in some cases longer than the diameter of the Milky Way.
The jets emitted from the centres of these active galaxies, called active galactic nuclei (AGNs), offer excellent conditions for particle acceleration to the highest energies and for the emission of gamma rays. AGN account for one-third of all known very high-energy (VHE) gamma-ray sources and are nearly the only objects we can detect at these energies that are not located in our own Galaxy.
The CTAO aims to measure large samples of such active galaxies, and galactic black holes, to study particle acceleration and gamma-ray emission processes. These observations will give us a picture of the conditions and physical processes occurring in and around some of nature’s most mysterious objects.

Supernova Remnants
When massive stars reach the end of their natural lifetime, they die in a gigantic explosion called a supernova. The explosion causes a large part of the stellar material to be expelled at thousands of kilometres per second into the surrounding interstellar environment. The resulting shock fronts are called supernova remnants (SNRs), which emit radiation across the whole electromagnetic spectrum and play an important role in the evolution of galaxies.
It is now known that charged particles can be accelerated by SNRs to reach energies beyond those achievable with the most powerful man-made particle accelerator, the Large Hadron Collider at CERN. SNRs may be the dominant source of the cosmic rays that bombard the Earth. Particles accelerated in SNRs are implicated in the growth of magnetic fields in the Universe and can influence star-formation in galaxies.
The CTAO will be able to detect a much larger number of SNRs in gamma rays than is currently possible and measure the properties of these objects in much greater detail, helping us to understand the process of particle acceleration in SNRs and the propagation of these particles away from SNRs and their subsequent impact on the interstellar medium. Crucially, for the first time, the CTAO will be able to probe particle acceleration up to Petaelectronvolt (PeV or 10^15 eV) energies in these objects and for any class of objects within our Galaxy. We know from the locally measured cosmic rays that something in our Galaxy accelerates particles to those energies, but the sources remain unknown. There is very recent evidence of particle acceleration in the Galactic Centre, but it is not clear if it can provide the local cosmic rays.

When a massive star reaches the end of its life, it undergoes a supernova explosion, ejecting most of its outer layers. The remaining core of the star collapses and, depending on its mass, becomes a white dwarf, a neutron star or a black hole. Neutron stars are formed from the collapse of ordinary stars roughly 8-20 times the size of the Sun and are incredibly dense – the equivalent of the Earth’s mass condensed into a space the size of 1-2 football stadiums.
In the collapse process, as the radius of the star decreases, the magnetic field becomes stronger and the rate of rotation increases (often rotating many times per second). As it rotates, so does its magnetic field, creating an electric field on the surface that accelerates charged particles. The radiation produced by these particles during their acceleration leads to a beam of electromagnetic emission along the axis of the magnetic field. As the neutron star rotates, the jets may swing past the Earth’s direction, much as the light from a lighthouse passes over the sea, leading to the observation of pulsed objects or “pulsars.”
The pulsar’s rotation rate slows down over time, as it uses its rotational energy to accelerate particles to high energies. These particles, trapped by the magnetic field, rotate in sync with the pulsar out to large distances. At a distance called the light cylinder, the particles would have to travel at the speed of light to continue keeping up with the pulsar. Rather than break the laws of physics, the particles are able to escape from the immediate region around the pulsar at this location, streaming away and creating what is called a pulsar wind. When this ultra-fast wind plows into the surrounding material, it creates a shock wave where particles are accelerated, spreading out into a cloud called a pulsar wind nebula (PWN).

Emissions from both pulsars and their wind nebulae have been detected at TeV energies. Pulsar wind nebulae (PWNe) are the most populous class of galactic objects in this energy range. The most famous PWN is the Crab Nebula, which formed from the Crab supernova explosion in 1054 AD, as recorded by Chinese astronomers. The Crab is one of the brightest TeV sources and was the first TeV gamma-ray source to be detected (in 1989). Pulsation from the Crab pulsar has been detected across the electromagnetic spectrum, from radio up to approximately TeV.
Observations with the CTAO will provide the first truly detailed gamma-ray images of PWNe and make it possible to probe the motion and cooling of high-energy particles in PWNe. The CTAO will also greatly increase the number of known PWNe, helping to further the understanding of their evolution and the impact of their environment. Additionally, the CTAO will provide insights in the central engine of PWNe, the pulsar itself, greatly expanding the number of known VHE pulsars and the precision with which they can be measured.

Binary Systems
Binary systems are composed of two objects that closely orbit one other, exchanging matter and energy through accretion processes or via the periodic interaction of their respective winds. Stars much more massive than the S un can have very powerful winds, which in star binary systems, collide and can accelerate particles, producing gamma-ray emission. In some cases, one of the companions of the binary system is a “compact object,” such as a black hole or neutron star, which pulls in material from the companion star through an accretion disk . From the vicinity of such compact objects, jets of particles that travel close to the speed of light can be emitted, giving rise to high-energy electromagnetic radiation. As a binary system moves through its orbit, the physical conditions in the collision region change. For this reason, binaries can be thought of as a laboratory for high – energy astrophysics, allowing scientists to adjust the parameters of the system and see what happens.
A few hundred of these systems have been discovered in our G alaxy thanks to the advent of X-ray astronomy in the 1960’s, but only a handful of binary systems emitting VHE gamma rays have been detected in our G alaxy in recent years. The improved capabilities of Cherenkov telescopes ( H.E.S.S. , MAGIC and VERITAS ) have made these more recent discoveries possible. Their discovery has proven to be extremely useful to study high-energy processes, in particular particle acceleration, emission and radiation reprocessing, and the dynamics of the underlying magnetized flows. The CTA O will greatly expand the population of gamma-ray binaries and allow us to precisely measure the behaviour of many systems as a function of orbital phase and photon energy. These measurements are expected to cast light on the physics of particle acceleration, as well as the winds of pulsars and massive stars and the way they interact.

Dark Matter
The nature of dark matter is one of the biggest outstanding questions in science. Dark matter is known to exist due to its gravitational effects and in far larger quantities than normal matter, but close to nothing is known about what it is. Many hypotheses exist for dark matter, mostly postulating a new weakly interacting massive particle, or WIMP). Some of the most promising theories predict that WIMPs can annihilate when they interact to produce more familiar particles. Such annihilations inevitably produce gamma rays.
There is a strong idea as to how often these annihilations should happen in order to give the WIMPs the right density in the Universe today, as well as where to look for this signal – in places where the density of dark matter is very high ( for example, the centre of our own G alaxy). Up until now, there have not been instruments sensitive enough to see the predicted signal. The CTA O will reach this critical sensitivity and complement other searches using the Fermi satellite, the L arge H adron C ollider (LHC) and deep underground direct searches for WIMPs. Together these instruments have a very good chance to solve the mystery of dark matter within this decade. More about how the CTAO will study dark matter.

Cosmic Voids
Most of the Universe is very close to empty, with matter clustered into galaxy clusters, super-clusters and filaments, separated by huge voids. How empty these voids are is a matter of great debate. In particular, are there any tiny magnetic fields in these regions that are a relic of the earliest moments of the Universe? The CTAO will be able to probe magnetic fields in the voids via observations of halos around active galaxies and also help to probe the proposed heating of low-density regions in the Universe by TeV photon interactions. A known ingredient of the space between galaxy clusters is the extragalactic background light, the integrated light of all processes over the history of the Universe in the infrared to ultraviolet range. The CTAO will be able to characterize these radiation fields via the absorption features that they leave in the spectra of the population of active galaxies seen by the CTAO.

Learn more about the topics the CTAO will be studying in Study Topics .
Il CTAO Organizza L’Evento Pubblico e Gratuito “DM dall’Universo” il 16 Aprile a Bologna
2024-march-25, lst-1 discovers the most distant agn at very high energies, 2023-december-26, the ctao will double its staff as major infrastructure development begins in 2024, 2023-september-25.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.2: The Electromagnetic Spectrum
- Last updated
- Save as PDF
- Page ID 85160

- Elizabeth Gordon
- Furman University
Learning Objectives
- Understand the mathematical relationship between frequency and wavelength.
- Recall the metric system of conversion.
- Correlate frequency or wavelength to energy (qualitatively).
- Calculate frequency or wavelength of radiation.
- Know the order of the electromagnetic spectrum (names, not numbers)
Roentgen, Becquerel and the Curies experimented with radiation. Using machines, Roengten's focused on the production X-rays, which is a wave-type of radiation. Unlike Roengten, Henri Becquerel studied the natural radioactivity of elements. His uranium experiments yielded the production of alpha (particle-type) and gamma (ray-type) radiations. Becquerel's two graduate students, Marie and Pierre Curie, isolated two radioactive elements. Both radium and polonium emit alpha and beta particles upon decay. In this section, wave form radiation will be explored. This type of radiation has an extremely wide range of wavelengths, frequencies, and energies.
Properties of Waves
A wave is a periodic oscillation that transmits energy through space . Anyone who has visited a beach or dropped a stone into a puddle has observed waves traveling through water (Figure \(\PageIndex{1}\)). These waves are produced when wind, a stone, or some other disturbance, such as a passing boat, transfers energy to the water, causing the surface to oscillate up and down as the energy travels outward from its point of origin. As a wave passes a particular point on the surface of the water, anything floating there moves up and down.
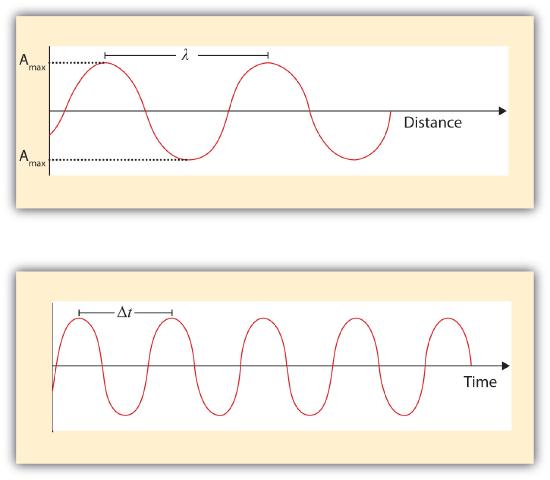
Waves have characteristic properties (Figure \(\PageIndex{1}\)). Waves are periodic, meaning, they repeat regularly in both space and time. The distance between two corresponding points in a wave—between the midpoints of two peaks, for example, or two troughs—is the wavelength ( λ ), distance between two corresponding pointsperiodween the midpoints of two peaks or two troughs. \(\lambda\) is the lowercase Greek lambda, and \(\nu\) is the lowercase Greek nu. Wavelengths are described by a unit of distance, typically meters. The frequency ( ν ) is the number of oscillations (i.e., of a wave) that pass a particular point in a given period of time . The unit for frequency is per second (1/s = s −1 ), which is equivalent to the SI unit of hertz (Hz). Amplitude, or vertical height of a wave, is defined as half the peak-to-trough. As the amplitude of a wave with a given frequency increases, so does its energy.
Electromagnetic Radiation
Energy that is transmitted, or radiated, through space in the form of periodic oscillations of electric and magnetic fields is known as electromagnetic radiation. (Figure \(\PageIndex{2}\)). Some forms of electromagnetic radiation are shown in Figure \(\PageIndex{4}\). In a vacuum, all forms of electromagnetic radiation—whether microwaves, visible light, or gamma rays—travel at the speed of light (c), which is the speed with which all forms of electromagnetic radiation travel in a vacuum , a fundamental physical constant with a value of 2.99792458 × 10 8 m/s (which is about 3.00 ×10 8 m/s or 1.86 × 10 5 mi/s). This is about a million times faster than the speed of sound.
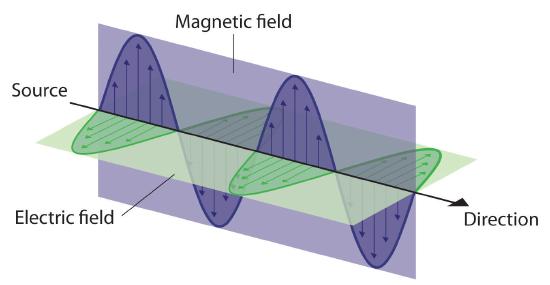
Because the various kinds of electromagnetic radiation all have the same speed ( c ), they differ in only wavelength and frequency. As shown in Figure \(\PageIndex{3}\) and Table \(\PageIndex{1}\), the wavelengths of familiar electromagnetic radiation range from 10 1 m for radio waves to 10 −12 m for gamma rays, which are emitted by nuclear reactions. Viewing the equation below, we can see how frequency of electromagnetic radiation is inversely proportional to its wavelength:
\[ \begin{array}{cc} c=\lambda \nu \\ \nu =\dfrac{c}{\lambda } \end{array} \label{6.1.2} \]
For example, the frequency of radio waves is about 10 8 Hz, whereas the frequency of gamma rays is about 10 20 Hz. Visible light, which is electromagnetic radiation that can be detected by the human eye, has wavelengths between about 7 × 10 −7 m (700 nm, or 4.3 × 10 14 Hz) and 4 × 10 −7 m (400 nm, or 7.5 × 10 14 Hz). Note that when frequency increases, wavelength decreases; c being a constant stays the same. Similarly when frequency decreases, the wavelength increases.
Please memorize equation 5.2.1 and the speed of light (with units). In addition, it is important to know which side of the electromagnetic spectrum is deadly.
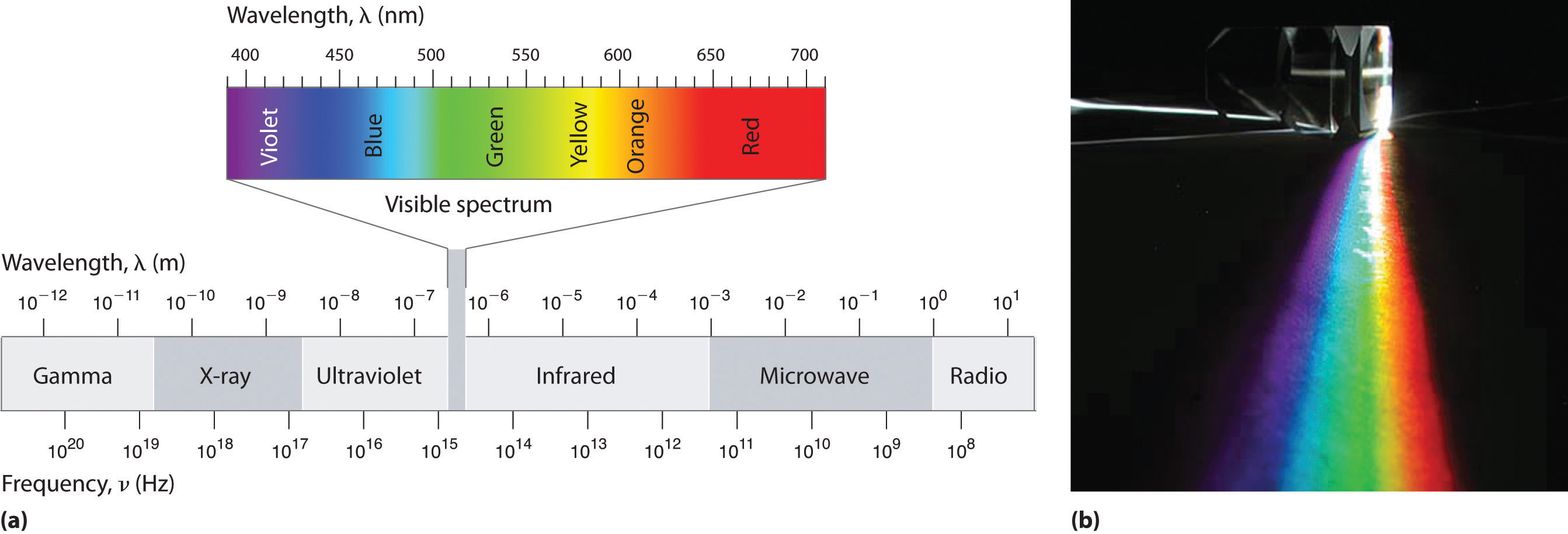
Light also behaves like a package of energy. It turns out that for light, the energy of the “package” of energy is proportional to its frequency.
\[ E\; \propto\; \nu \label{6.1.3} \]
\[ E\; \propto\; \dfrac{1}{\lambda } \label{6.1.4} \]
Whereas visible light is essentially harmless to our skin, ultraviolet light, with wavelengths of ≤ 400 nm, has enough energy to cause severe damage to our skin in the form of sunburn. Because the ozone layer absorbs sunlight with wavelengths less than 350 nm, it protects us from the damaging effects of highly energetic ultraviolet radiation.
In this course, we will not do energy calculations. You should know the relationship between frequency and energy. Also, you show realize that short wavelength radiation is associated with a high energy.
The energy of electromagnetic radiation increases with increasing frequency and decreasing wavelength.
Example \(\PageIndex{1}\)
What is the frequency of light if its wavelength is 5.55 × 10 −7 m?
We use the equation that relates the wavelength and frequency of light with its speed. We have
\[3.00\times 10^{8}m/s=\left ( 5.55\times 10^{-7} m\right)\nu \nonumber \]
We divide both sides of the equation by 5.55 × 10 −7 m and get
\[\nu =5.41\times 10^{14}s^{-1} \nonumber \]
Note how the m units cancel, leaving s in the denominator. A unit in a denominator is indicated by a −1 power—s −1 —and read as “per second.”
Exercise \(\PageIndex{1}\)
What is the wavelength (in mm) of light if its frequency is 1.55 × 10 10 s −1 ?
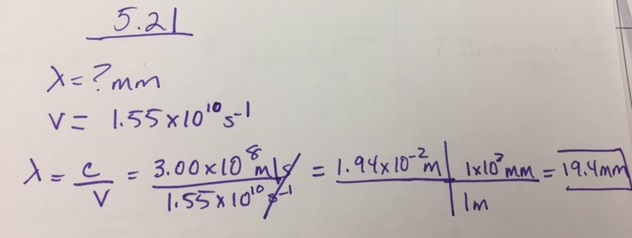
Example \(\PageIndex{2}\)
Calculate the frequency of radiation if its wavelength is 988 nm. Where does this radiation appear in the electromagnetic spectrum?
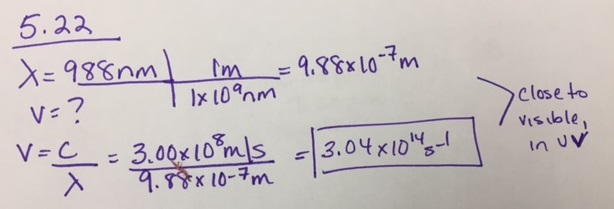
Need More Practice?
- Refer to section 5.E of this OER and work problem #4. Refer to the electromagnetic spectrum to locate the type of radiation.
Contributors and Attributions
Elizabeth R. Gordon (Furman University)
- Emma Gibney (Furman University)
Faster-Than-Light Travel Could Explain Mysterious Signals Beaming Through the Cosmos
But don't worry, no laws of physics are being violated.
In a distant corner of the universe, something is traveling faster than light.
No, the laws of physics aren't being violated: It's still true that nothing can travel faster than light in the vacuum of empty space. But when light travels through matter , like interstellar gas or a soup of charged particles, it slow downs, meaning other matter might overtake it. And that may explain the weird symmetry in pulses of some of the most energetic light in the universe, called gamma-ray bursts.
Related: 8 Ways You Can See Einstein’s Theory of Relativity in Real Life
These cryptic bursts — bright flashes of gamma-ray light that come from faraway galaxies — form when massive stars collapse or when ultradense neutron stars collide. These cataclysms send speeding jets of hot, charged plasma zooming through space.
But these signals have an odd symmetry , and the reason they do is still a mystery.
A gamma-ray burst doesn't brighten and dim in one steady peak, but instead in a flickering pattern, said Jon Hakkila, an astrophysicist at the College of Charleston in South Carolina.
Hakkila has worked on this puzzle for years. Now, he and a collaborator have a solution: plasma traveling both slower and faster than the speed of light could explain this flickering pattern, as they report in a paper published Sept. 23 in The Astrophysical Journal . If they're right, it may help us understand what's actually producing these gamma-rays.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"I find it a great step forward," that connects the small scale phenomena in the plasma to our large-scale observations, said Dieter Hartmann, an astrophysicist at Clemson University who was not involved in the study.
In the last few years, Hakkila has found that gamma-ray bursts have small fluctuations in brightness on top of their overall brightening and dimming. If you subtract the overarching brightening and dimming, you're left with a series of smaller peaks — one primary peak with smaller peaks in brightness before and after. And this pattern is strangely symmetric. If you "fold" the pattern over at the main peak and stretch one side, the two sides match remarkably well. In other words, the light pattern of a gamma-ray burst's pulse hints at a set of mirrored events.
"Whatever happened on the front side happened on the back side," Hakkila said. "And the events knew to happen in reverse order."
Though astronomers don't know what causes gamma-ray burst emission at the particle scale, they are fairly sure that it happens when jets of plasma traveling near the speed of light interact with surrounding gases. Hakkila had been trying to come up with explanations for how these situations might make symmetric light pulses when he heard from Robert Nemiroff, an astrophysicist at Michigan Technological University.
Nemiroff was studying what happens when an object travels through a surrounding medium faster than the light it emits, called superluminal motion. In previous research, Nemiroff had found that when such an object goes from traveling slower than light to faster than light, or vice versa, this transition can trigger a phenomenon called relativistic image doubling. Nemiroff wondered whether this could account for the symmetric patterns Hakkila found in gamma-ray burst pulses.
So what exactly is "relativistic image doubling?" Imagine a boat creating ripples as it moves across a lake toward the shore. If the boat travels more slowly than the waves it creates, a person standing on the shore will see the boat's ripples hit the shore in the order that the boat created them. But if the boat travels faster than the waves it creates, the boat will overtake the first wave it creates only to create a new ripple in front of that one and so on. In that way, the new ripples created by the boat will reach the shore sooner than the first waves it created. A person standing on the shore will see the ripples hit the shore in a time-reversed order.
The same idea applies to gamma-ray bursts. If the cause of a gamma-ray burst is traveling faster than the light it emits through the gas and matter surrounding it, we would see the emission pattern in reverse chronological order.
Hakkila and Nemiroff reasoned that this could account for half of a gamma-ray burst's symmetric pulse.
But what if the material was first traveling slower than the speed of light, but then accelerated? What if it started fast and then slowed down? In either case, we might see the emission both in chronological order and reverse chronological order right after one another, making a symmetric pulse pattern like the symmetric peaks observed in gamma-ray bursts.
There are still missing pieces to this puzzle. For one, researchers still don't know what's causing these bursts at the microscopic scale. But this proposed model gives researchers one small clue in the hunt to find the ultimate cause of gamma-ray bursts, Hartmann said.
Originally published on Live Science .
- The 7 Silliest Time Travel Concepts in Science Fiction
- The 12 Strangest Objects in the Universe
- The Biggest Unsolved Mysteries in Physics
2024 solar eclipse map: Where to see the eclipse on April 8
NASA jets will chase the eclipse at 460 mph on Monday. Here's why.
Longest eclipse ever: How scientists rode the supersonic Concorde jet to see a 74-minute totality
Most Popular
By Elise Poore April 05, 2024
By Keumars Afifi-Sabet April 05, 2024
By Ben Turner April 05, 2024
By Sascha Pare April 05, 2024
By Patrick Pester April 05, 2024
By Daisy Dobrijevic April 05, 2024
By Joanna Thompson April 05, 2024
By Brandon Specktor April 04, 2024
By Ben Turner April 04, 2024
By Nicoletta Lanese April 04, 2024
By Jonathan Gilbert April 04, 2024
- 2 Error-corrected qubits 800 times more reliable after breakthrough, paving the way for 'next level' of quantum computing
- 3 April 8 solar eclipse: What time does totality start in every state?
- 4 Giant coyote killed in southern Michigan turns out to be a gray wolf — despite the species vanishing from region 100 years ago
- 5 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 2 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 3 'Gambling with your life': Experts weigh in on dangers of the Wim Hof method
- 4 Cholesterol-gobbling gut bacteria could protect against heart disease
- 5 NASA engineers discover why Voyager 1 is sending a stream of gibberish from outside our solar system

What’s Made in a Thunderstorm and Faster Than Lightning? Gamma Rays!
A flash of lightning. A roll of thunder. These are normal stormy sights and sounds. But sometimes, up above the clouds, stranger things happen. Our Fermi Gamma-ray Space Telescope has spotted bursts of gamma rays – some of the highest-energy forms of light in the universe – coming from thunderstorms. Gamma rays are usually found coming from objects with crazy extreme physics like neutron stars and black holes . So why is Fermi seeing them come from thunderstorms?
Thunderstorms form when warm, damp air near the ground starts to rise and encounters colder air. As the warm air rises, moisture condenses into water droplets. The upward-moving water droplets bump into downward-moving ice crystals, stripping off electrons and creating a static charge in the cloud.
The top of the storm becomes positively charged, and the bottom becomes negatively charged, like two ends of a battery . Eventually the opposite charges build enough to overcome the insulating properties of the surrounding air – and zap! You get lightning.
Scientists suspect that lightning reconfigures the cloud’s electrical field . In some cases, this allows electrons to rush toward the upper part of the storm at nearly the speed of light. That makes thunderstorms the most powerful natural particle accelerators on Earth!
When those electrons run into air molecules, they emit a terrestrial gamma-ray flash, which means that thunderstorms are creating some of the highest energy forms of light in the universe. But that’s not all – thunderstorms can also produce antimatter ! Yep, you read that correctly! Sometimes, a gamma ray will run into an atom and produce an electron and a positron, which is an electron’s antimatter opposite!
Fermi can spot terrestrial gamma-ray flashes within 500 miles (800 kilometers) of the location directly below the spacecraft. It does this using an instrument called the Gamma-ray Burst Monitor which is primarily used to watch for spectacular flashes of gamma rays coming from the universe.
There are an estimated 1,800 thunderstorms occurring on Earth at any given moment. Over its first 10 years in space, Fermi spotted about 5,000 terrestrial gamma-ray flashes. But scientists estimate that there are 1,000 of these flashes every day – we’re just seeing the ones that are within 500 miles of Fermi’s regular orbits, which don’t cover the U.S. or Europe.
The map above shows all the flashes Fermi saw between 2008 and 2018. (Notice there’s a blob missing over the lower part of South America. That’s the South Atlantic Anomaly, a portion of the sky where radiation affects spacecraft and causes data glitches.)
Fermi has also spotted terrestrial gamma-ray flashes coming from individual tropical weather systems . In 2014 Tropical Storm Julio produced four flashes in just 100 minutes!
Related Terms
- Black Holes
- Extreme Weather Events
- Fermi Gamma-Ray Space Telescope
- Gamma-Ray Bursts
- Neutron Stars
- The Universe
- Weather and Atmospheric Dynamics
Explore More

NASA’s New Hubble E-Book Spotlights Universe’s Best-Kept Dark Secrets

Hubble Peers at Pair of Closely Interacting Galaxies

How NASA’s Roman Telescope Will Measure Ages of Stars
Guessing your age might be a popular carnival game, but for astronomers it’s a real challenge to determine the ages of stars. Once a star like our Sun has settled into steady nuclear fusion, or the mature phase of its life, it changes little for billions of years. One exception to that rule is the […]
Discover More Topics From NASA
Dark Matter & Dark Energy

The Big Bang
Which travels faster in a vacuum - gamma rays or infrared rays?
In a vacuum, they both travel the exact same speed, the speed of light. IIRC that's about 3x108 meters per second.
Add your answer:
Which travels faster in a vacuum gamma rays or infrared rays?
Neither. In vacuum, all electromagnetic radiation has the same speed, regardless of wavelength. It's the speed we call "the speed of light", but it applies to all of those other electromagnetic phenomena too.
Which has higher energy infrared light or gamma rays?
The energy of gamma radiation is much higher than the energy of infrared radiation. You are emitting infrared radiation, but gamma radiation is very harmful to living tissue.
What gives off more energy infrared waves or gamma rays?
In a vacuum do different parts of the electromagnetic spectrum travel at the same or different speeds.
In vacuum, every imaginable wavelength of electromagnetic radiation, from longer than radio waves to shorter than gamma rays, travels at 299,792,458 meters per second.
Which travels faster radio waves or microwaves?
-- Microwave ARE radio waves.-- All electromagnetic waves travel at the same speed, including radio, microwaves,heat, infrared radiation, light, ultraviolet radiation, X-rays, gamma rays, and allthe others.
Which type of wave travels faster gamma or radio?
The same. Both are electromagnetic waves; in a vacuum, they both travel at the speed of light.
Why is infrared light faster than ultraviolet light?
That would be a very difficult "why" to explain, because it doesn't. All light travels at the same speed in the same medium. In vacuum, it's the speed we call "The Speed of Light". Shockingly, it's the same as the speed of radio, TV, radiant heat, X-rays, gamma rays, WiFi, and microwaves.
What is the difference between infrared ray and gamma rays?
The main difference between gamma rays and infrared rays is in their wavelengths. Gamma rays have the shortest wavelengths while infrared rays have longer wavelengths. Gamma and infrared rays are types of electromagnetic radiation.
What other form of energy travels at the speed of light?
All electromagnetic radiation does. That includes radio, microwave, infrared, ultraviolet, X-rays, gamma rays, etc. ... stuff like that.
Do Cell Phones Use Gamma-Electric Technology?
no infrared
Which has the greatest frequency out of radio wave microwave infrared wave visible light ultraviolet light x ray gamma ray?
X-rays have the highest frequency in the electromagnetic spectrum.
Gamma rays have shorter wavelength than infrared rays?
Yes. The wavelength of radiation is w=hc/Energy. Gamma energy is larger than infrared energy, thus has shorter wavelength.
How do infrared waves compare with gamma rays?
Much lower frequency.
Why have scientists put ultraviolet infrared infrared gamma's and X ray telescopes in space?
they did it to get more detailed pictures of space
Top Categories


IMAGES
VIDEO
COMMENTS
But last year, researchers found events in some gamma-ray burst pulses that seemed to repeat themselves as though they were going backwards in time. Now, new research suggests a potential answer for what might be causing this time reversibility effect. If waves within the relativistic jets that produce gamma-ray bursts travel faster than light ...
Light of any wavelength, from picometer-wavelength gamma-rays to radio waves more than a trillion times longer, all move at the speed of light in a vacuum. The frequency of any photon is equal to ...
Gamma rays have the highest frequency, whereas radio waves have the lowest. ... of energy that are lower in frequency (and thus longer in wavelength) than visible light. These types of energy include infrared (IR) rays (heat waves given off by thermal bodies), microwaves, and radio waves. These types of radiation surround us constantly, and are ...
In that section, it was pointed out that the only difference between radio waves, visible light and gamma rays is the energy of the photons. Radio waves have photons with the lowest energies. Microwaves have a little more energy than radio waves. Infrared has still more, followed by visible, ultraviolet, X-rays and gamma rays.
Ultraviolet radiation falls in the range from a few electron volts to about 100 eV. X-ray photons have energies in the range 100 eV to 100,000 eV (or 100 keV). Gamma-rays then are all the photons with energies greater than 100 keV. Show me a chart of the wavelength, frequency, and energy regimes of the spectrum. Why do we put telescopes in orbit?
Light of any wavelength, from picometer-wavelength gamma-rays to radio waves more than a trillion times longer, all move at the speed of light in a vacuum. The frequency of any photon is equal to ...
The primary source of infrared radiation is heat. The higher the temperature, the faster the atoms and molecules in an object move and the more infrared radiation. The first infrared space mission was IRAS (Infrared Astronomical Satellite) which detected about 350 000 infrared sources. ... Gamma rays (wavelengths less than 0.01 nanometres)
Gamma rays have wavelengths shorter than 10^-11 meters and frequencies above 30 x 10^18 hertz. The European Space Agency describes how gamma-ray photons have energies in excess of 100,000 ...
To see the gas clouds between galaxies, we need X-ray detectors. We've been using telescopes designed to reveal the invisible parts of the cosmos for more than 60 years. Because Earth's ...
Gamma-ray wavelengths are so short that they can pass through the space within the atoms of a detector. Gamma-ray detectors typically contain densely packed crystal blocks. As gamma rays pass through, they collide with electrons in the crystal. This process is called Compton scattering, wherein a gamma ray strikes an electron and loses energy ...
Infrared Radiation. The boundary between the microwave and infrared regions of the electromagnetic spectrum is not well defined (see Figure 16.17). ... Gamma rays have characteristics identical to X-rays of the same frequency—they differ only in source. The name "gamma rays" is generally used for electromagnetic radiation emitted by a ...
The electromagnetic spectrum consists of gamma rays, X-rays, ultraviolet radiation, visible light, infrared, and radio radiation. Many of these wavelengths cannot penetrate the layers of Earth's atmosphere and must be observed from space, whereas others—such as visible light, FM radio and TV—can penetrate to Earth's surface.
And it implies the more energy a photon has the faster it moves. I don't understand the whole light is photons and waves at the same time thing, but I thought the speed of light was constant, that Gamma rays travel at the same speed as visible light, infrared, microwaves, etc.
The gamma rays CTAO will detect are about 10 trillion times more energetic than visible light. The electromagnetic spectrum provides scientists with a variety of ways to view the Universe. As seen in the figures below, telescopes detecting different frequencies of light provide different perspectives of the cosmic sources, such as the Milky Way ...
$\begingroup$ The wavelengths of radio waves are actually in the range $10^{-3}$ to $10^5$ m, whereas those of gamma rays are less than $10^{-11}$ m. As mentioned in one of the answers, $700$ nm and $400$ nm are the wavelengths of red and violet visible light respectively. $\endgroup$
published 29 April 2019. The Vela pulsar that lives 1,000 light years from our planet.(Image credit: NASA/CXC/Univ of Toronto/M.Durant et al) Charged particles travel faster than light through the ...
Now, recent research suggests a potential answer for what might be causing this time reversibility effect. If waves within the relativistic jets that produce gamma-ray bursts travel faster than light - at 'superluminal' speeds - one of the effects could be time reversibility. Such speeding waves could actually be possible.
In a vacuum, all forms of electromagnetic radiation—whether microwaves, visible light, or gamma rays—travel at the speed of light (c), which is the speed with which all forms of electromagnetic radiation travel in a vacuum, a fundamental physical constant with a value of 2.99792458 × 10 8 m/s (which is about 3.00 ×10 8 m/s or 1.86 × 10 5 ...
Yet in space many strange things happen, including a new proposal by two astrophysicists that blasts creating bursts of gamma rays may be able to speed up faster than light, going superluminal.
The same idea applies to gamma-ray bursts. If the cause of a gamma-ray burst is traveling faster than the light it emits through the gas and matter surrounding it, we would see the emission ...
So why, then, was there a recent story claiming that gamma-ray jets, where gamma-rays themselves are a high-energy form of light, can travel faster-than-light? That's what Dr. Jeff Landrum wants ...
These events, called terrestrial gamma-ray flashes, last less than a millisecond and produce gamma rays with tens of millions of times the energy of visible light. Thunderstorms form when warm, damp air near the ground starts to rise and encounters colder air. As the warm air rises, moisture condenses into water droplets.
Yes. The wavelength of radiation is w=hc/Energy. Gamma energy is larger than infrared energy, thus has shorter wavelength.