
Find Healthcare
Programs & services, public health postnatal services.
Location: Three Hills Health Centre
Services are offered to parents who:
- have been discharged with their baby
- have been discharged while their baby is still in the hospital
- are adopting
- have chosen adoption for their baby
- had a stillborn baby
- are dealing with loss due to a neonatal (newborn) death
Public health nurses contact families within 48 hours after discharge from the hospital. Services are offered according to the situation and discussion with the family and may include health assessments of the newborn, mother and family; information about what to expect and when to seek medical attention; feeding support and referrals to community services.
Three Hills Health Centre
Monday 8:30 am - 4:30 pm
Tuesday 8:30 am - 4:30 pm
Wednesday 8:30 am - 4:30 pm
Thursday 8:30 am - 4:30 pm
Friday 8:30 am - 4:30 pm
Closed from 12:00 PM to 1:00 PM for Lunch.
Parent who delivered a baby and / or experienced perinatal loss.
Parent(s) with babies from birth to 2 months.
If you have not been contacted by a public health nurse within 48 hours after discharge, phone your local Community Health Centre. Contact the Community Health Centre for information and support.
- Camrose Community Health Centre Briarcrest
- Castor Community Health Centre
- Consort Community Health Centre
- Coronation Community Health Centre
- Drayton Valley Community Health Centre
- Drumheller Health Centre
- Eckville Community Health Centre
- Elnora Community Health Centre
- Hanna Health Centre
- Innisfail Health Centre
- Kitscoty Community Health Centre
- Lacombe Community Health Centre
- Lamont Health Care Centre
- Olds Campus Community Health Centre
- Ponoka Community Health Centre
- Provost Provincial Building
- Red Deer Johnstone Crossing Community Health Centre
- Rimbey Community Health Centre
- Rocky Mountain House Health Centre
- Sedgewick Community Health Centre
- Stettler Community Health Centre
- Sundre Community Health Centre
- Sylvan Lake Community Health Centre
- Tofield Health Centre
- Two Hills Health Centre
- Vegreville Community Health Centre
- Vermilion Provincial Building
- Wainwright Provincial Building
- Wetaskiwin Community Health Centre
Contact Details
Related info.
Please enable javascript to use this website
Updating app (1 / 2)

Our Philosophy
We have created this clinic with the intention of families feeling heard, respected, and comforted knowing that they can birth their babies into an environment of peace, instead of fear. We believe in the incredible design of our bodies and the miracle of pregnancy and birth.
The foundation of our practice is based on informed choice, informed consent, bodily autonomy and respect. We feel that it is an honour to journey with you and your family through your pregnancy, labour, birth and postpartum period.
Our Services
We offer personalized, professional, respectful care for birthing families.

Prenatal Care
Regular prenatal care helps to keep mothers and babies safe and healthy. It is also a time where the midwives and families get to know each other... Read More >>

Labour and Birth Care
During labour a midwife will be with you to help encourage and support you. When you get closer to having the baby a second midwife will come and you will... Read More >>
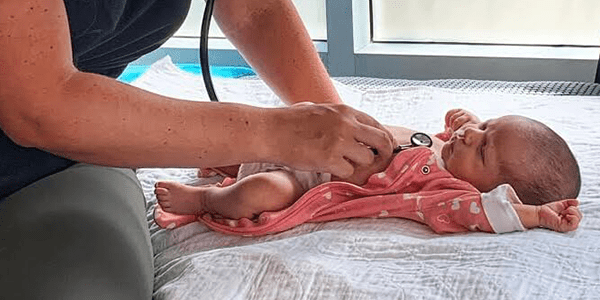
Postnatal Care
Approximately 3-4 home visits will be done in the first week postpartum, depending on individual needs. At the home visits we monitor mother and... Read More >>
Resources and information for pregnancy and postpartum
Testimonials.
We look forward to welcoming you.

- Pager: 403-214-7557 Opt.1 *pager does not accept text messages
- Email: [email protected]
- Address: #1121 - 403 Mackenzie Way SW, Airdrie AB, T4B 3V7
Should public health nurses visit every family with a new baby?
Sara Savidant already had two children, but that didn’t make the Lethbridge, Alberta mom feel any more confident when she was caring for newborn twins in 2015. “I forgot so much in that short amount of time between the births of my kids,” she says.
But when the public health nurse walked in the door, she immediately felt at ease. “She just offered me reassurance,” recalls Savidant, who invited the nurse to visit a few more times. “It felt different from seeing a doctor, I felt more comfortable in my own home.” Plus, it was extremely difficult for Savidant to get to the clinic – she was recovering from a C-section and couldn’t carry one car seat, let alone two. So she was relieved the nurse could examine her twins at home. (Savidant ended up taking one of the twins to a doctor, because the nurse was concerned about jaundice.)
In most jurisdictions across Canada and many higher-income countries, public health nurses follow up with women after delivery through phone calls and/or home visits, to assess their needs, recommend community programs and give advice on feeding, safety, early childhood development and more.
“You really get a much more natural sense of how things are going for them in home visits,” explains Barbara Webster, a clinical nurse specialist who used to do home visits and now oversees community nurses at the First Nations Health Authority in BC. “We can talk about the safety things that are pertinent in the house (such as potential suffocation hazards in a crib), and the woman doesn’t have to get out of her pajamas.”
But depending on the province and region moms live in, they either get a lot, or very little, public health follow up. As to the best way to conduct the visits, how many should be provided, and who should be visited, that’s a question health officials are still trying to figure out. Many argue, however, that the current follow up is inadequate.
“Sometimes it seems like we provide all this prenatal support, and the baby is born, and we say, ‘Wonderful! Congratulations! Good luck!’ It’s almost like saying, ‘We don’t do returns,’” jokes Michael Geary, chief of obstetrics and gynecology at St. Michael’s Hospital. “But an adverse environment in the first year of life has been shown to affect your ability to thrive and grow well into adulthood…we really need to focus more on the first weeks, months and year of life.” Indeed, poor early nutrition, inadequate cognitive stimulation and other issues in infancy have been shown to increase the risk of poor long-term health outcomes , such as cardiovascular disease, diabetes, obesity and more.
What’s the evidence behind postpartum home visits?
Home visits have been shown to increase mother-child interactions and positive stimulation for babies . Systematic reviews have found nurse visiting led to greater uptake of other medical and educational interventions (such as pediatric visits and community support groups) as well as improved the safety of the home environment . One randomized controlled trial found nurse visits decreased smoking rates and slightly improved cognitive development among babies, among other effects.
A review of six studies in which women were provided home visits with counselors trained in postpartum depression found that four of them demonstrated that the visits helped prevent or improve postpartum depression.
But not all studies have noticed differences between families visited by public health nurses and those not visited. Generally, the effects are found when the mothers are considered high-risk , based on how they answer screening questions, or because they are young, or have low levels of education, income or family support. When home visits are provided to everyone regardless of need, benefits are often unnoticeable .
In fact, one study randomized 733 women to either receive a telephone call (and be visited if the nurse or mother felt there was a need) or to be visited at home twice, without an assessment. The study found no difference between maternal confidence nor health problems in infants in each group at four weeks. Similarly, there was no difference between the two group’s breastfeeding rates at six months. But the cost difference between the two interventions was significant, with the cost of the call group averaging about $150 per infant and the two-visit group averaging $240 per infant.
Geary points out, however, that if the public health interventions are dramatically helpful for a very small percentage of the general population, that can be hard to capture in a study. And it’s necessary to reach everyone in order to reach the few who will be helped, he argues. This is why all women receive home visits in the UK , where Geary did his medical training.
“We’re very good at identifying the high-risk mom and baby and managing them, but the real challenge is identifying the high risk baby of the low-risk mom,” he says. (In other words, sometimes, babies born to families who have high levels of resources and supports still have issues such as undernourishment. And those babies could be missed because no problems are expected.) Additionally, when it comes to postpartum depression, which can have devastating effects on moms and babies if left untreated, the benefits of the home visits are noticed among studies of the general population.
It’s important to note that not all public health programs are created equally. The effects of home visits are greatest when the visits are intensive and frequent, as well as combined with a larger program. One of the most frequently cited studies showed impressive impacts of home visits among high-risk women in Elmira, New York. There, women were visited weekly for at least eight weeks (half the women were additionally followed up to 24 months) and were provided with transportation to medical appointments.
In comparison, an Ontario study found there was no difference in breastfeeding rates between women who were called or visited by a public health nurse, and those who were not – possibly because most women only received one home visit, on average.
Public health follow up across Canada
While some jurisdictions have moved to reduce the number of moms who get public health follow-up home visit(s), others have been expanding the number. This year, for example, Toronto Public Health (TPH) changed its policy from only following up with women who screened as at-risk based on their answers to an in-hospital questionnaire. Now, they’ll be calling all women who fill out the questionnaire and consent to have their information shared with public health, regardless of their risk level. They’ll then visit those who indicate in the phone call that they could benefit from a home visit.
Lynn Walker, manager of Child Health & Development, Reproductive and Infant Health at TPH, says the change was made based on “anecdotal feedback” from families saying they appreciated the call, and it helped them get connected to local services such as breastfeeding clinics.
In 2012, the province of BC moved in the opposite direction , but ended up with the same intervention as TPH now employs. Previously, all BC women were visited in their homes; now all women are called and those who ask for are deemed to need a visit are visited.
Tama Cross, a midwife with Diversity Midwives in Scarborough, Ontario, agrees with the phone call-first approach. “We have quite a few clients who say ‘I’m fine, I don’t need the extra [public health support],’” she says. Still, she thinks it would be better if women are called a second time, perhaps after a couple of weeks, as postpartum depression and other issues may not set in immediately.
In Alberta, meanwhile, all women have a comprehensive assessment with a public health nurse to cover everything from domestic violence screening, postpartum depression screening, breastfeeding support, and general health assessments of the mother and baby. In the Calgary and Edmonton zones, these assessments often take place in clinics, while outside of these zones, most women are seen in their homes.
Because the public health nurses combine the mental and psychosocial assessment of the mother with the examination of the child, this universal approach doesn’t end up costing significantly more than a more selective approach, explains Shannon Evans, a spokesperson for Alberta Health Services. “The psychosocial health of the parent is as important to the child as it is to the parent,” she adds.
How many visits a woman receives, and what programs she’s referred to, also varies. In Alberta, a woman who is determined as needing more support may be visited weekly for up to two months; in Ontario, in very high-risk cases, families can be visited until a child is six years. Usually, however, a family that isn’t facing significant challenges will only get one visit.
Ways to improve home visits of families with newborns
Moira Sherman, a mother of two in Toronto, suffered from postpartum depression after both of her births and was visited by public health nurses both times. When her second was born, she asked specifically about postpartum depression programs, but the two programs in her area weren’t focused on depression in particular, but overall mother-child interaction. Meanwhile, the nurse didn’t offer counseling herself, but “extremely basic” advice about tummy time and feeding. “I’m a second-time mom; it felt like amateur hour,” says Sherman.
Toronto Public Health does offer free programs specific to postpartum depression , but the programs are only available in certain neighbourhoods. “Since all Healthy Baby Healthy Child programs are funded by the province, there should be more consistency in what is offered,” says a Greater Toronto Area public health nurse who wishes to remain anonymous.
Peter Spadoni, a media spokesperson for Ontario’s Ministry of Children and Youth Services says the current program is under review — in large part to address the inconsistencies with some jurisdictions (like TPH’s) choosing to call all women, while others only call at-risk families.
As to what can be done about the wide variability in community programs that nurses refer women to, each public health unit decides individually what programs it will fund. However, Patients First legislation , expected to be reintroduced this fall, may address the potential inequities by providing Local Health Integrated Network (LHIN) oversight of public health planning and programming.
There’s also concern that nurses aren’t targeting socioeconomically disadvantaged women enough. In one Ontario study , despite being 2.5 times more likely to request a public health nurse home visit, socioeconomically disadvantaged women only received one half visit more, on average, than socioeconomically advantaged women over the first four weeks post birth.
Part of the issue may be a communication failure. A 2013 evaluation of the Healthy Babies, Healthy Children program found that 18% of postpartum women weren’t reached for a phone assessment, despite having screened as having risk and consenting to having their information shared. The anonymous GTA nurse says many high-risk moms don’t have voicemail or money for minutes to call a nurse. “If you need to change the visit or you want to reconnect with someone, there’s that inability to connect,” she says.
One way to address the problem is to allow nurses to text clients, the nurse suggests. Additionally, more community workers can be engaged at the local level. In many jurisdictions, trained home visitors who are not nurses conduct follow-up visits in consultation with a nurse, and these visitors could be utilized to locate women who may be in need. For women who may not be comfortable having someone visit in their home, jurisdictions in several provinces also offer assessments at local community centres.
For First Nations people in BC, having home visitors from the local communities has helped tremendously in ensuring all women are visited, says Lucy Barney, a perinatal specialist with the First Nations Health Authority. “They often already have a relationship with the family, so they know when they’re coming home with the baby,” she says.
Cross agrees that more community supports and more visits in general are needed for the largely socioeconomically disadvantaged population she sees. “I see a lot of isolation, a lot of postpartum depression,” says Cross. “They’re asking for more help in the home, more contact, more resources.”

Wendy Glauser
Contributor
Wendy is a freelance health and science journalist and a former staff reporter with Healthy Debate.
Michael Nolan
Michael Nolan has served Canadians through many facets of Paramedic Services. He is currently the Director and Chief of the Paramedic Service for the County of Renfrew and strategic advisor to Healthy Debate
Jeremy Petch
Jeremy is an Assistant Professor at the University of Toronto’s Institute of Health Policy, Management and Evaluation, and has a PhD in Philosophy (Health Policy Ethics) from York University. He is the former managing editor of Healthy Debate and co-founded Faces of Healthcare

Republish this article on your website under the creative commons licence.
The comments section is closed.
Very telling that there is NO information whatsoever on how to opt out of these home visits. I don’t need domestic violence counseling, birth control counseling, don’t need or want a stranger other than our family doctors examining me or my child, and don’t want or need any of these other services. If I do, I will get them on my own. I understand why the services are offered, and that many women need them, but when I know I don’t I find the questions about domestic violence, my private life, etc., nosy and intrusive. My doctor already knows I will say OPT OUT when she starts on them. I have searched endlessly for a way to opt out, and all I find are sites with glowing reviews from happy mothers, gushing about how worthwhile this is and EVERYBODY NEEDS TO DO IT!!! without information on how to say a polite NO THANK YOU, WE ARE FINE, SO PLEASE SAVE YOUR TIME FOR SOMEBODY WHO DOES NEED AND WANT IT.
Nearly all the mothers on the sites I asked told me it’s required by law, that I cannot decline any of the services (including exams and tests on me!), and some even said Child Protection caseworkers would come and take away my baby if I even let up so much as a peep to object “because it’s a big red flag!” THIS IS JUST WRONG.
Some of us have had more than one baby already, some of us have hired home nannies, others have plenty of support and prefer to keep their privacy – like me.
YOU NEED AN OPT OUT INSTEAD OF THIS UNDERLYING BULLYING THAT YOU ARE BY GOD COMING TO OUR HOUSE AND WE BETTER LIKE IT.
Please note that I am asking for instructions to opt out. I am NOT asking for a discussion about how worthwhile this is or why you think I need to comply. Thank you.
Do we get the choice to refuse visits if we do not want them. Im not comfortable with people I don’t know in my home. I would feel much MUCH more comfortable going to a clinic instead of feeling intruded upon
@Alicia, the answer is yes. Most maternal child home visiting programs are voluntary and a mother, parent, caregiver who is a referral has to agree to be seen by the home visiting program. Also, consent forms must be signed prior to being enrolled in these type of nurse home visitor programs. Maternal and infant death doesn’t discriminate. Which means wealthy, highly educated, normal relationship mothers/ families have experienced Loss of a loved one in this “status quo class” during their pregnancy, postpartum as well as this same group of people have experienced the death an infant before its 1st birthday.
I am SO grateful I had a visit from the public health nurse after my daughter was born. If it wasn’t for her, I would not have known my daughter was even tongue tied. The “lactation consultant” I saw in the hospital didn’t even bother to check when I was having problems nursing. The public health nurse also directed me to a breastfeeding moms support group, with nurses and a lactation consultant in attendance every week. Without that group, I would have given up on nursing after a week probably, and they were able to answer so many other questions I had as a new mom. The public health nurse was so kind and helpful, and followed up with me 2 more times after that initial visit. Every mom should have access to this if they choose.
Do public health nurse visits moms and babies from out of country that were discharged from postpartum?
Bringing a new baby home is a majorlife event even though it has been anticipated for many months. As a former PHN and IBCLC I can cite many many examples of the value of that home visit witin the first 24 hrs. We started the Early Discharge program with the knowledge that mother and baby would be supported by a home vist. This made the early discharge program possible. If we stop doing the home visits then early discharge is no longer safe for the mother and baby. The many reasons for designing the post part/newborn program with an early home bisit are too numerous to cite. It is all about health promotion and prevention rather than waiting for illness to occur with a re admission to hospital. The burden on the new family is too great.
Such a circular argument. Huge self selection bias. Those who are “fine” and “don’t need help” will say no because this isn’t a social norm here like it is in the countries with much better child health outcomes. (Nordic countries.) Meanwhile many of these same moms are in their doctors office or talking with their friends about how hard it all is – how they never learned emotional self regulation but now have a screaming baby, or a baby not sleeping, or tantrums, or, or, or. And they are trying to figure out how to cope, how to not yell or hit because they know they feel terrible after and they know there is a better way. Is home visiting all figured out yet? no. Is it imperative that we figure it out and get it right? Only if we care about the future, the roots of violence, and healthy brain, body and life development. Public programs are phenomenal, even quality child care under age 2 is impactful. But NOTHING has as much impact as the home environment, the family relationships, and the dynamics at home.
Yet another example of public health busybodies wanting to interfere with peoples lives and wag their fingers saying “we know best”. Plus the self-interest they have in seeking to expand their budgets, their importance and their salaries through some form of universal program.
A good friend of mine (well educated, wealthy, in a stable relationship) said yes to a home visit from a public health nurse after my friend had her baby. That nurse commented about everything that was wrong, wrote up a report and did follow up visits without asking permission. My friend felt like she was being judged by the public health police and now there is a file about her held by the government.
it is time we learned in health care that more “care” for everyone may not make sense. It is perhaps better to target the care to those who need/want it, more vulnerable etc and leave it all well alone. Plus we need better coordination of care across silos – no point having public health home visits if there’s no coordination with primary care physicians/providers or other providers to the family.
So Adam, would your friend be able to identify if her baby had jaundice? Or some other condition her baby could DIE from? Just because some one is well educated, wealthy and in a stable relationship doesn’t mean they can identity issues with their baby. Further more is your friend able to assess herself for post Partum depression? Is she able to understand the hormone imbalances her body will go through? Maybe your friend is well educated, wealthy and in a stable relationship but she is not a public health nurse who had been trained for years to identify problems which mothers and babies died from even as little as 10 years ago. If you are okay with babies dying than yes you are right public health officials should mind their own business
It is unfortunate that your friend had that experience because her in Northern Colorado we have a program that is very successful. The two nurses use a very non-judgemental approach and are truly there to help these moms be successful. They assist with getting resources if needed, sign up for Medicaid, sustain breastfeeding if that is their goal, and so much more. They build a relationship that it motivated by the mother’s goals and needs. The only priority of the program is to have the mothers feels supported and to succeed.
My niece just had a baby 3 weeks ago and she is going through hell how do we go about getting someone in to help her
Republish this article
- Please use the invisible republishing code below on the page where you republish this article.
- Please give credit to Healthy Debate and include a link back to our home page or the article URL . Our preference is a credit at the top of the article and that you include our logo (available by clicking the link below).
Please read the full set of instructions for republication here .
Language selection
- Français fr
Chapter 5: Postpartum Care
- Previous Chapter
- Table of Contents
- Next Chapter

Download in PDF format (5.54 MB, 85 pages)
Organization: Public Health Agency of Canada
Date published: 2020-12-16
Related Topics
- Chapter 5 Fact sheet: Family-Centred Postpartum Experience
- Chapter 5 Infographic: Postpartum Health in Canada
- Family-Centred Maternity and Newborn Care: National Guidelines
- Fact sheets and infographics: Maternity and newborn care
Acknowledgements
Introduction, 1.1 cultural considerations, 1.2 caring for indigenous women, newborns and their families, 1.3 caring for lgbtq 2 families, 2.1 integrated care of the mother and baby, 2.2 care of the mother, 2.3 care of the newborn, 3.1 hospital births: length of hospital stay, 3.2 care of the mother, 3.3 care of the newborn, 4.1 postpartum mental health, 4.2 late postpartum hemorrhage, 4.3 infections, 4.4 cardiovascular and hypertensive disorders of pregnancy, 4.5 extensive perineal tears, 4.6 female genital mutilation/cutting (fgm/c), 4.7 diastasis of the rectus abdominis muscle, 4.8 gestational diabetes mellitus (gdm), 4.9 thyroid conditions, 4.10 symphysis pubic dysfunction, pelvic girdle pain and diastasis symphysis pubis, 4.11 assisted vaginal birth, 4.12 urinary/fecal incontinence, 4.13 prolonged stay in hospital, 5.1 infections, 5.2 cardiorespiratory distress and cardiac concerns, 5.3 hypoglycemia, 5.4 prenatal antidepressant use, 5.5 small-for-gestational-age babies and macrosomia, 5.6 substance use – neonatal abstinence syndrome/neonatal adaptations syndrome, 5.7 late preterm babies, 5.8 assisted vaginal birth, 5.9 anomalies or rare conditions, 5.10 prolonged stay in hospital/neonatal intensive care unit (nicu), 6.1 systems to follow families postpartum, 6.2 ongoing postpartum care of the mother and baby, 6.3 intimate partner violence and child abuse, 6.4 mother's nutrition and healthy weight, 6.5 sexuality and contraception, 6.6 immunization, lead author, georgia hunt, md.
Assistant Head, Quality Department of Family Practice BC Women’s Hospital Vancouver, British Columbia
Contributing Authors
Angela bowen, rn, phd.
Professor College of Nursing University of Saskatchewan Saskatoon, Saskatchewan
Christina M. Cantin, RN, BScN, MScN, PNC(C)
Perinatal Consultant Champlain Maternal Newborn Regional Program Ottawa, Ontario
Beverley Chalmers, DSc(Med), PhD
International Perinatal Health Consultant Kingston, Ontario
Kimberly Dow, MD, FRCPC
Professor Department of Pediatrics Queens University Kingston, Ontario
Louise Hanvey, RN, BScN, MHA
Senior Policy Analyst Maternal and Child Health Public Health Agency of Canada Ottawa, Ontario
Faiza Khurshid, MBBS, FCPS, MSc(HQ)
Assistant Professor, Department of Pediatrics Queen's University Medical Director, Division of Neonatal-Perinatal Medicine Kingston Health Sciences Centre Kingston, Ontario
Céline Lemay, SF, PhD
Senior lecturer Bac en pratique sage-femme Université du Québec à Trois-Rivières Trois-Rivières, Québec
Tracy Lovett, RN, BScN, MN, IBCLC
Maternal Child Health Nurse Coordinator First Nations and Inuit Health Branch, Atlantic Region Indigenous Services Canada Halifax, Nova Scotia
Lynn M. Menard, RN, BScN, MA
Team Leader Maternal and Child Health Public Health Agency of Canada Ottawa, Ontario
Simone Vigod, MD, MSc, FRCPC
Chief, Department of Psychiatry, Women’s College Hospital Associate Professor and Director, Department of Psychiatry, Faculty of Medicine, University of Toronto Shirley A. Brown Memorial Chair in Women’s Mental Health Research, Women’s College Hospital Toronto, Ontario
Carley Nicholson, RD, MPH
Policy Analyst Maternal and Child Health Public Health Agency of Canada Ottawa, Ontario
Lori E. Ross, PhD
Associate Professor and PhD Program Director Division of Social and Behavioural Health Sciences Dalla Lana School of Public Health, University of Toronto Toronto, Ontario
Roberta Stout
Research Associate National Collaborating Centre for Indigenous Health Prince George, British Columbia
The postpartum period is a significant time for the mother, baby, partner, and family. It is a time of transition and adaptation and is formative for everyone. There are physiological adjustments for both mother and baby, and significant social and emotional adjustments for the entire family.
Complex and finely tuned adjustments have physical and psychological benefits for the mother and her baby. It is important that everyone involved in the care of mothers and babies knows and acknowledges these benefits so that systems are planned and organized around the mother/baby unit and not around health care providers (HCPs).
The goals of care during the postpartum period are to:
- Support and promote the physical well-being of mother and baby and enable the mother to restand recover from the physical demands of pregnancy and birth;
- Support the developing relationship between the baby and their mother as well as the mother’s partner and family;
- Support the mother’s and her partner’s emotional and mental health needs;
- Support infant feeding;
- Support the mother’s confidence in herself and in her baby’s health and well-being, enabling her to fulfill her mothering role within her particular family and culture; and
- Support partners and other family members to enable them to develop confidence in their new role.
According to the principles of family-centred care, it is incumbent on HCPs to:
- Treat families with respect, dignity, and kindness, and learn about and respect their values and beliefs, using them to guide their care;
- Maintain open and ongoing communication with the woman and her partner/family;
- Plan the timing and purpose of each postpartum contact in partnership with the woman and her partner/family based on their individual needs;
- Provide culturally competent and safe care with cultural humility;
- Provide information and support in a timely fashion, according to the needs of the woman, her partner, and family. Ensure that information is evidence-based and accessible according to their culture, language, and abilities so that they can promote their own and their baby’s health and make informed decisions about their care and any necessary treatment.
Additional resources on postpartum care see Appendix A
1. Families with Special Considerations
Canadians are ethnoculturally diverse. Women from different cultures, whether Canadian-born or newcomers, may be influenced to a greater or lesser extent by their background.
HCPs will want to assess each woman’s background—if they are newcomers, their place of birth, how long they have been in Canada—and their support networks. Footnote 1 It is important to understand how the woman’s culture influences her unique needs, hopes, and postpartum expectations. Even when the necessary services are available and they are made aware of them, immigrant women may face language barriers and difficulties in access because of differences in cultural practices and expectations. Footnote 2
Most women who are newcomers to Canada face challenges of some sort: Footnote 2
- The Canadian health care system may feel foreign and strange, and they may have different expectations from those of their HCPs.
- They may not know about the available supports in the health care system.
- They may not share a common language with available HCPs, and their communities may not have access to culturally sensitive health care or translation services.
Each family is unique; they adapt their cultural traditions and practices to their own experience and needs, and they will interpret the parameters of the Canadian health care system within this context. As such, it is important to assess each situation individually. While HCPs may not always agree with certain cultural practices, respecting families’ needs and decisions is paramount. Listening to the women’s and families’ stories about their own culture, childbearing practices, and needs helps accomplish this approach. Footnote 1 Giving Birth in a New Land: Strategies for service providers working with newcomers suggests specific strategies that promote family-centred, culturally competent postpartum care. Footnote 3 HCPs can use these to engage in a dialogue with women and families and learn about their values and beliefs and how these apply to their situation.
Questions to Facilitate Communication about Values and Beliefs Footnote 1 Footnote 3
If families are newcomers to Canada, ask about their place of birth, how long they have been in Canada, and their support systems. To ensure that women have an opportunity to express their needs, helpful questions include:
- How is health care different in your homeland or culture?
- What do you and your family believe you should do to remain healthy postpartum?
- What are the things you do to improve your health and the health of your baby? What can’t you do?
- Do you have beliefs about caring for your baby and yourself that I need to know about?
- Do you have any practices and faith rituals to do with your role as a mother?
- Are there any specific foods that you might eat/drink (or prefer to avoid)?
- Are there any home remedies that you may use during the postpartum period?
- Who do you want involved in decision-making?
Adapted from Giving Birth in a New Land: Strategies for service providers working with newcomers (2014), and Maternal Child Nursing Care Canada (2017).
HCPs need to consider the personal values they bring to their relationship with families. Cultural competence includes working collaboratively with families and communicating effectively.
Communication with families from different cultural backgrounds involves not only translating words, but also understanding subtle variations in meaning, style, volume, and gestures. Footnote 1 As such, it is important to find the best possible interpreter for the specific situation. Interpreters must be trustworthy regarding access to private information and, ideally, have specific health-related language skills. Using children or other family members as interpreters is not recommended.
The history of residential schools and colonization, which caused the loss of traditional values and practices, languages, and family/community kinship, continues to affect Indigenous women, families, and communities. Indigenous Peoples have poorer health outcomes and higher rates of poverty, food insecurity, and unsafe and overcrowded housing. These social determinants of health take a toll on the physical, emotional, mental, and spiritual health of Indigenous women in Canada.
The health and well-being of many Indigenous women and families have been further undermined by racism, sexism, and culturally inappropriate or inaccessible health services—which also affect Indigenous women and their babies during the postpartum period. Footnote 4 Footnote 5 Footnote 6
Indigenous women in Canada are diverse in their culture, ancestry, beliefs, and practices. Each Indigenous community has its own traditions, values, language, and communication styles. Many Indigenous women want to incorporate their cultural and societal values and beliefs into their lives and parenting. Integrating cultural safety in the care of Indigenous women during the postpartum period involves providing an environment of respect and open communication, which is consistent with the principles of family-centred care. Indigenous women, as all women, need to feel safe in order to build a trusting relationship with their HCPs.
HCPs should engage with, and familiarize themselves with, the community and work with women to understand their individual values, beliefs, and needs. Footnote 7
An Indigenous doula can assist in honouring traditional and spiritual practices and beliefs associated with postpartum care and support the woman and her family’s language and cultural needs while providing emotional and physical assistance during pregnancy, labour, and the postpartum period. Footnote 8
Indigenous women may have to leave their communities to give birth in larger centres. Being away from their families and support systems affects their postpartum experience, including breastfeeding, and recovery. It is important to consider their needs and re-connect them with families and communities as quickly as possible.
Indigenous-specific postpartum and parenting programs are ideal—particularly group formats that allow Indigenous women to meet each other and develop supportive friendships during their pregnancies. Programs that support Indigenous fathers so that they feel equipped to help their partners and children are also necessary. Better systems of referrals and communication between different services and organizations would ensure continuity and comprehensiveness in care. Footnote 9
Training and Education of Healthcare Providers
There is a need for better training of HCPs on how to create culturally safe, stigma-free, and respectful care for Indigenous mothers, babies, and families during the postpartum period. Footnote 9 A nationwide survey of residents and program directors of all accredited obstetrics and gynecology residency programs in Canada demonstrated a lack of curriculum and a significant deficit in knowledge in Indigenous women’s health. Footnote 10 As a result, a nationwide curriculum initiative is underway for residents and other health care practitioners. This will facilitate the provision of education in Indigenous women’s health while decreasing the burden on individual programs.
Family-centred maternity and newborn care is based on individual needs and a mutually respectful and trusting relationship. While progress has been made in providing equitable health care to the LGBTQ 2 community Footnote * , these families often continue to face barriers in health care.
People in the LGBTQ 2 community identify 3 major barriers when dealing with the health care system—invisibility, lack of information, and negative beliefs. Invisibility refers to the fact that they do not see themselves in the institutions/programs—for example, the posters on the walls, the forms they complete—or in conversations with HCPs. The HCPs they encounter often do not understand their experiences as an LGBTQ 2 family, their unique and diverse needs, and may have negative beliefs about them. Footnote 11
Sexual minority women (including lesbian, bisexual, and other non-heterosexual women) have a greater prevalence of depression and depressive symptoms compared with heterosexual women, likely because of the impact of sexual orientation-based discrimination, stigma, lack of social support and exposure to additional stress due to heterosexism from their families and some HCPs. Footnote 12 Footnote 13 Footnote 14 Footnote 15 Footnote 16 Invisible sexual minority women (i.e., women who have a history of sexual relationships with women but are currently partnered with men) are at higher risk for postpartum depression than both visible sexual minority women (women partnered with women) and heterosexual women. Footnote 14 Footnote 17
Ongoing education for HCPs on the unique needs of LGTBQ2 families is essential to improving the health care LGTBQ2 families receive. Footnote 18 HCPs caring for LGBTQ 2 families will want to confront any negative beliefs they may have and aim for ease in approaching the topics of gender, sexuality, and families. It is important that HCPs reflect on their beliefs about LGBTQ 2 people and be willing to challenge these beliefs to develop their practice. Footnote 11
HCPs can facilitate inclusivity when caring for LGBTQ 2 families, including during the postpartum period, by: Footnote 11
- Paying attention to words and language. Words can empower people and they can hurt.
- Being aware of non-verbal communication and tone of voice—these express emotions and attitudes.
- Using non-biased, inclusive language and open-ended questions.
- Asking questions that express openness to all families—and not making assumptions about gender identity, sexual orientation, or behaviour.
- Making sure forms and questionnaires are inclusive.
- Ensuring that visuals, such as posters, in a clinic or program area signal acceptance of diversity.
- Posting a non-discrimination policy and communicating an environment of respect.
2. Postpartum Care Immediately After Childbirth (Birth to 2 Hours)
The mother and newborn should be considered a unit during the immediate postpartum period (0–2 hours). It is important to avoid disrupting this close relationship during these crucial few hours and to encourage skin-to-skin contact between the baby and the mother (or partner if the mother is unable). The International MotherBaby Childbirth Organization refers to this as motherbaby care to emphasize the importance of recognizing that mothers and babies are a unit.
The parent–baby bond—the first step in the baby’s subsequent attachments—is formative to a child’s sense of security and has long-lasting effects. Footnote 19 Having early physical contact with the baby can affirm parents’ sense of accomplishment and promote their self-confidence as parents. Keeping babies and parents together is of the highest priority. Institutional policies should only disrupt this contact in the event of a necessary, evidence-based medical reason.
All major organizations concerned with newborn health, including the Society of Obstetricians and Gynaecologists of Canada (SOGC), the Breastfeeding Committee for Canada, the Canadian Paediatric Society (CPS), the Canadian Association of Midwives, the American Academy of Pediatrics, the World Health Organization (WHO), and the United Nations Children’s Fund (UNICEF), recommend that healthy babies have direct skin-to-skin contact with their mothers immediately following birth. Skin-to-skin contact involves placing the newborn babies on their mothers’ bare chest immediately after she gives birth, covering the baby with a blanket, and ensuring that contact is uninterrupted for at least an hour or at least until the first feeding is completed or the mother wishes. Footnote 20 It is essential to prepare mothers for skin-to-skin contact before birth. Since some cultures may not practise this contact, information, encouragement and support are called for.
Being held by their mother helps the baby normalize his or her temperature, breathing, heart rate, and blood sugar and reduces the pain of medical procedures. Babies who have skin-to-skin contact interact more with their mothers and cry less than those who do not have this contact. Footnote 21 Footnote 22 Footnote 23 The vast majority of babies go to the breast within an hour of birth if they are kept skin-to-skin with their mother. Mothers are more likely to breastfeed in the 4 months postpartum and tend to breastfeed for longer if they have early skin-to-skin contact with their babies. Footnote 22 Nevertheless, skin-to-skin contact is important for all mothers and babies regardless of the mother’s decision about feeding. If the mother herself is unable to have skin-to-skin contact with her baby, she should choose another person to hold, warm, and comfort the baby, for example, her partner or another family member.
HCPs can demonstrate respect for the family by interfering as little as possible during interactions between the mother and baby. Observations, assessments, and interventions can be completed with minimal intrusion, while skin-to-skin contact is maintained. Anything that is not essential to the immediate well-being of the baby or mother can wait for 2 hours or after the first breastfeeding. Even medically necessary procedures can be done while the baby remains in skin-to-skin contact as long as it is medically safe to do so. Footnote 23 Footnote 24
Skin-to-skin contact should continue during transfer from the birthing unit to the postpartum unit or neonatal intensive care unit (NICU). Footnote 25 At this time, babies should be observed for abnormal respiratory effort, colour, activity or tone—signs of instability that call for urgent evaluation. Separating a mother from a baby requiring special care can make adjustment to motherhood more difficult, and HCPs are called upon to provide even more intensive support at such times. There are continued benefits to skin-to-skin contact past the immediate first few hours of birth, as well as benefits to initiating skin-to-skin contact later, if this was not possible immediately following birth. Footnote 23 Footnote 24 Footnote 26
In some Canadian and European centres, preterm babies stay with their parents during assessments, and couplet care is practised within the NICU. Footnote 27 Many centres are advocating for skin-to-skin contact, even of very preterm, ventilated, and low birth-weight babies, because of the clinical and psychological benefits to both baby and parents. Footnote 28
Family-Integrated Care in the Neonatal Intensive Care Unit
Recent Canadian research has found that a family-integrated care (FICare) model of care for preterm babies in neonatal intensive care units (NICU) is feasible and safe in the Canadian health care setting and results in improved weight gain by these babies. The FICare model of care, which is based on the original work of Dr. Adik Levin in Estonia, also has the potential to improve other short- and long-term outcomes for babies and families. Footnote 29
In this model, parents provide most of the care for their baby, while nurses and other HCPs guide and counsel parents. Footnote 29 Footnote 30 FICare is more than just the physical setting; the model recognizes that parents are the primary caregivers and decision-makers for their babies. FICare can be accomplished even in older units, and HCP teams are expected to adapt to that reality whenever possible.
Innovative examples of this model of care in Canada include the following:
- BC Women's hospital offers intensive care for newborns and postpartum care for mothers in the same room. Mothers are able to recover from vaginal or caesarean births and pump breast milk without leaving their babies. All newborn babies have their own sound-proofed rooms, and 12 of the 70 rooms are spacious mom-and-babyrooms equipped with a breast-pumping station, reclining chair, and hospital bed for the mother as well as an incubator and infant-monitoring machines. The mom-and-baby rooms are for babies born at 33 weeks or later at low risk of complications.
- In Nova Scotia, the IWK Health Centre is caring for mothers and babies together in their NICU. Each room has a full setup to care for a baby as well as a suite for the family to stay in. The family is given a double bed, a closet with a safe, and a private washroom with a shower. Babies are continuously monitored and, if an alarm is triggered, a signal is sent to a nurse’s smartphone. The rooms are also equipped with everything from milk fridges to special sinks that help families bathe their babies. Rooms without windows have skylights that mimic clouds in the sky, and every room has artwork.
In the event of a caesarean birth, it is important to provide all possible opportunities for immediate (defined as within 5 minutes) and uninterrupted skin-to-skin contact as well as breastfeeding when babies cue to feed. This can be done in the operating and recovery rooms. In fact, skin-to-skin care should be considered the norm for caesarean births in the operating room, decreasing the need for early supplemental feedings. Footnote 31
It is important to provide time alone for the family in those critical first hours, with opportunities for both parents to interact with the baby in the birth and recovery rooms. Parents should be encouraged to spend as much time as possible with their baby, including in the NICU, ideally while rooming-in together. If the woman’s partner chooses not to be present for the caesarean birth, the family should be re-united as soon as possible. Footnote 32
The immediate postpartum period is a time of joyful celebration for the vast majority of families, but it is also a time of considerable physiological adaptation for the mother—and for the baby. As such, careful observation and, at times, intervention is required.
Women have different responses on giving birth. Some feel excited, uplifted, and energetic. Others are exhausted and want to sleep. A woman’s response may depend on the length, difficulty, and pain during labour, blood loss, anesthesia/analgesia, complications, and whether she had an operative vaginal birth or caesarean birth. Another determining factor is the woman’s experience of labour and birth compared with her expectations of these events.
Physical adjustments in the immediate postpartum period—including blood loss, weight loss, and displacement of internal organs—require a significant expenditure of energy. Immediate postpartum care centers on the need for hydration, nutrition, and rest. It is a time to replenish energy.
Begin each postpartum contact by asking the woman how she feels, physically and emotionally, and identifying any concerns that she may have. The physical observation of the mother at each postpartum contact should be individualized and guided by her unique history and situation. The assessment can include the following, depending on the mother’s feelings, sensations, and expressed needs: Footnote 24
- Vital signs (temperature, pulse, respiratory rate, blood pressure);
- Uterine tone and condition of perineum;
- Bladder and bowel function;
- Breasts and nipples;
- Physical comfort;
- Emotional and psychological response to labour and birth, for the woman and her partner. Starting this conversation is particularly important in certain circumstances (e.g., when the baby is sick, the mother had complications, or the birth did not go as planned);
- Skin-to-skin contact with baby; and
- Learning needs.
Document the findings according to the institution’s policy.
Postpartum Hemorrhage
Postpartum hemorrhage is the most common complication in the immediate postpartum period. It affects approximately 6% of women globally and is the leading cause of maternal mortality worldwide. Footnote 33 In Canada, a diagnosis of postpartum hemorrhage was associated with 1.6 maternal deaths per 100,000 hospital births from 2002 to 2010. Footnote 34 From 2006 to 2010, it was the second most common severe maternal morbidity, at a rate of 465.4 per 100 000 hospital births. Footnote 34
Postpartum hemorrhage is defined as blood loss of more than 500 mL during vaginal birth or more than 1000 mL during caesarean birth. The primary cause of immediate postpartum hemorrhage is uterine atony. Other causes include uterine rupture, morbidly adherent placenta, and uterine artery extension/laceration during caesarean birth.
Refer to the Society of Obstetricians and Gynaecologists of Canada (SOGC) guideline Active Management of the Third Stage of Labour: Prevention and treatment of postpartum hemorrhage . Footnote 35
Postpartum hemorrhage has many implications for the woman, including orthostatic hypotension, anemia, fatigue, and fear—all of which affect her ability to care for herself and her baby. It may also result in a lack of immediate skin-to-skin contact with her infant and an increase in the risk of postpartum depression. A blood transfusion may be necessary, which has risks. Footnote 36 Footnote 37 Delayed, or secondary, postpartum hemorrhage (between 24 hours and 6 weeks postpartum), may occur after the woman and baby have been transferred to a postpartum unit or at home.
It is important to educate women of the signs and symptoms of concern relating to delayed postpartum hemorrhage before discharge and after a homebirth.
The baby’s transition to life outside the uterus involves:
- Establishment of effective respiration and circulation;
- Maintenance of an adequate body temperature;
- Contact with his/her mother and family; and
- Initiation of feeding.
The postpartum period is a critical transition time for the baby. This period requires thorough and ongoing assessment and monitoring. An initial, head-to-toe examination of the baby in the birthing area ensures that he or she is adapting to the extrauterine environment. This examination would also identify any abnormal clinical findings. These observations can be completed when the baby is skin-to-skin, which promotes intimacy while helping to maintain a calm environment.
Neonatal Resuscitation
The Neonatal Resuscitation Program (NRP) acknowledges that at least 90% of newborns are vigorous, term babies who do not need to be separated from their mothers for the initial steps of resuscitation. Care for these babies includes:
- Managing the umbilical cord (i.e., avoidance, where possible, of immediate clamping);
- Providing warmth by encouraging direct skin-to-skin contact, ideally with the mother;
- Drying the baby’s skin with a warm, dry towel, stimulating breathing, and repositioning the head to open the airway;
- Clearing mucus from the upper airway, if necessary, by wiping the baby’s mouth and nose; and
- Ongoing observation of breathing, heart rate, activity, and colour. Footnote 38
Refer to NRP guidelines for the management of specific clinical situations. Footnote 23 Footnote 39
HCPs obtain skills in neonatal resuscitation through NRP training coordinated by the CPS, which has set the educational standards for Canadian practice. The Society recommends that an individual trained in neonatal resuscitation be assigned to this role at every birth. The CPS also recommends that all personnel likely to care for babies at birth have training and registration at the Provider or Instructor level and undergo periodic re-registration. Footnote 39
While the primary care provider at the birth is responsible for the woman’s care, a second HCP should have the primary role of assisting the baby through transition – one able to provide positive pressure ventilation and perform chest compressions, if necessary. Footnote 38 Another person with the skills to perform a complete resuscitation (including intubation and chest compressions) should be readily available to assist. Footnote 38 Footnote 40
The CPS also advises that local/regional health authorities have in place a program that supports the implementation of current neonatal resuscitation guidelines, educational programs for HCPs involved in care during labour and birth, and policies that take into account the educational needs, roles, and responsibilities of professionals involved in care during labour and birth/care of the newborn.
Neonatal Stabilization
A proportion of newly born babies are identified as at risk or unwell during the minutes or hours following birth, often due to prematurity or poor cardiorespiratory transition. All delivering facilities and practitioners should have a plan that addresses these babies’ clinical needs (such as respiratory support or glucose management), communication with referral centres, and support of the family.
The CPS’s Acute-Care of at-Risk Newborns (ACoRN) program specifically addresses the needs of babies who are challenged by the transition to extrauterine life. Facilities may find this program useful in preparing for the possibility that a newborn is unwell or at risk.
3. Early Postpartum Care (After 2 Hours)
The key goals of early postpartum care are to:
- maintain and promote the health and well-being of mother and baby;
- support the mother in caring for herself and her baby;
- foster attachment between the baby and the mother, her partner, and other significant family members;
- support the physical and psychological adjustment of the mother and her partner, the baby, and the family; and
- promote effective feeding.
Every postpartum interaction should be carried out in accordance with the principles of family-centred care, basing care and support on evidence of individual needs and not routines.
The benefits of skin-to-skin contact continue through the early postpartum period, facilitating attachment, increasing the duration of breastfeeding, and decreased crying and expression of pain during procedures such as heel prick blood sampling. Footnote 23 Although no national guidelines on labour, birthing, and postpartum rooms exist, the Provincial Council for Maternal and Child Health (PCMCH) recommends that mothers who give birth in hospital have a spacious room, preferably a private one, where they can labour, give birth, and stay with their babies until discharged. Rooming-in 24 hours a day should be the norm for all mother–baby dyads unless there is a justifiable reason for separation. Footnote 20 As many interventions as possible should occur in the mother’s room to avoid separation. Admissions to nurseries should be based on established criteria and guidelines—and be the exception rather than the rule. Footnote 23
A personalized postpartum care plan should be developed in partnership with the mother and her family as soon as possible following the birth. It includes: Footnote 24
- the mother’s concerns and needs;
- important factors in the pregnancy, birth, and immediate postpartum period;
- assessment of infant feeding;
- the names and contact information of the professionals involved in the mother’s and baby’s care; and
- planned follow-ups/appointments with HCPs for mother and baby during the postpartum period.
The plan needs to be reviewed and adjusted with the mother and family after every postpartum interaction.
Each mother should be assigned an HCP who is responsible for coordinating the care of the family and their transition into the community. This HCP consults with others, as necessary, as the needs of the mother and baby evolve. When birth takes place in hospital or a birthing centre, it is critical that systems, policies, and protocols ensure families are discharged only after follow-up care in the community is established. Footnote 41 Footnote 42
Optimal family-centred care during the early postpartum period requires seamless continuity of care and information-sharing between HCPs. How this is accomplished depends on the type of provider and the jurisdiction. Successful coordination of early postpartum care depends upon clear communication between institutions, community HCPs, and families. Hospitals, birth centres, physicians, nurse practitioners, and midwives need a strategy to facilitate effective communication of health information as mothers and babies transition into the community. A comprehensive discharge summary or maternal–newborn passport program may be useful. Secure electronic communication facilitates this process. Footnote 43
The Breastfeeding Committee of Canada and WHO recommend assessing newborn babies for breastfeeding issues within 24 to 48 hours of discharge from a hospital/birthing centre with routine follow-up of all mothers within 48 hours of discharge; Footnote 20 Footnote 44 this care may be provided by the hospital, community health centre, a breastfeeding clinic, midwife, etc.
Most newborn care guidelines recommend that an HCP assess the mother and baby during the first week of life. Footnote 45 The American Academy of Pediatrics specifies that this assessment takes place 48 to 72 hours after discharge if discharge occurs less than 48 hours following birth. Footnote 46 The CPS states:
At time of discharge, infants must have an appropriate follow-up plan in place that includes: contact information for a primary health care provider; a scheduled follow-up visit 24 h to 72 h post discharge—in hospital, clinic or at home—with a qualified health care provider. Hearing and newborn screens have been scheduled (if they were not conducted in-hospital); appropriate follow-up for jaundice; vitamin D supplementation if breast-fed; other follow-up, as required. Footnote 45
Even though the same principles and philosophy of care underpin all postpartum care, postpartum services should be organized locally to maximize effectiveness and efficiency for women and their babies.
In 1993, the average length of stay after a vaginal birth was 3.2 days, decreasing to 2.0 days by 2012. Footnote 5 During the same period, the length of hospital stay following Caesarean birth decreased from 5.0 days to 3.4 days. The safety of a shortened hospital stay (averaging 2.2 days in 2017/2018 Footnote 47 ) has been debated with regards to the needs of the mother and particularly the newborn. What research says about shorter hospital stays can differ from various organizations’ guidelines for both mother and baby. Footnote 48 Footnote 49
Each family needs to discuss with their HCP the risks and benefits of a stay that is shorter than the institutional standard. Base this discussion on the baby’s and the mother’s needs and not on routine policies. From the perspective of family-centred care, leaving the hospital as early as possible has a number of potential benefits: the opportunity for the entire family to get to know the baby together, resulting in greater attachment; more involvement for the partner and less sibling rivalry; better rest and sleep for the mother in her own environment, without constant interruptions from hospital staff; reduced exposure of mother and baby to hospital-acquired infections; and greater confidence on the mother’s part in her ability to care for her baby. Footnote 49
A shortened hospital or birthing centre stay is favoured by: the physiological stability of the mother and baby; family readiness to care for the baby at home; and a greater level of community, family, and institutional support upon discharge. In all situations, including those where mothers and babies are discharged early, mothers need to understand the signs of potential problems. In addition, it is important that the family knows where and when the mother and baby will next see an HCP and who they can contact with any questions.
The Canadian Medical Protective Association (CMPA) recommends reviewing test results and looking for signs of postpartum complications (e.g., infection, hemorrhage, excessive pain, bladder distention, difficulty walking) before discharging the mother and baby. The family should receive clear written or verbal instructions describing the steps and precautions to take when there are concerns, as well as the symptoms or signs that indicate that further medical attention is necessary. Footnote 50
Women and families should be told about community programs for postpartum care and peer supports for themselves and their babies—what they are, where they are located, and how to access them. These may include home-visiting programs, clinics, community-based programs and telephone support. Since it may be difficult for new mothers to remember all of the information shared with them, it is best to provide written information and also make it available on the facility’s website.
Regularly reviewing communication and coordination mechanisms will help to ensure a consistent and effective transition into the community and follow-up for the mother, baby, and family. The question of how best to arrange mother and baby’s discharge is an opportunity to revisit institutional and community resources for new families.
Refer to the following CPS guidelines related to infant discharge:
- Facilitating Discharge from Hospital of the Healthy Term Infant
- Safe Discharge of the Late Preterm Infant
- Going Home: Facilitating discharge of the preterm infant
At-risk babies, including those born late preterm babies, or those who are low birth-weight, are at risk for multiple complications including poor feeding and weight gain, hypoglycemia, and jaundice. Discharge should only be considered once the baby is stable.
Care and support during the early postpartum period should enable the mother to take charge of her own health and that of her baby—and to become confident in her ability to care for herself and her baby. This assumes that she is an autonomous adult and that HCPs have confidence in her ability to be a partner in her own care. Her values, situation, and needs are unique.
The Mother’s Well-being and Needs
Begin each postpartum contact with the mother and family by asking the woman how she feels, physically and emotionally, and identify any concerns she may have. Topics to explore include her experiences with her baby, breastfeeding/feeding, how much rest she is getting, and any pain or discomfort she may be experiencing. A physical examination may be performed as needed. The mother’s care is aimed at maintaining her health and helping her adapt to her new role as a mother.
Women need information, advice, and reassurance about postpartum physiological adaptations—such as normal lochia, perineal healing, incision healing (following caesarean birth), and changes to the breasts and nipples. They also need information on any potential issues, such as infection, hemorrhoids, cramping, constipation, urinary incontinence, painful urination, perineal pain and hygiene, headaches, back pain, pain medication, anemia, late postpartum hemorrhage, separation of the abdominal muscles, and breastfeeding challenges. The emotional and social changes she is likely to experience as a result of becoming a parent also require discussion.
HCPs will want to provide clear and consistent information and advice that is tailored to the woman’s individual needs and concerns. If the woman has a partner, he or she is a central figure in the family and should take part in conversations with consideration given to his or her needs. The psychosocial context of some situations may require particular attention, for example, support for single mothers, mothers in difficult socioeconomic situations, mothers who are new to Canada or who are refugees, mothers with psychosocial concerns identified during pregnancy, or teen mothers.
Mother’s Adjustment and Emotional Health
Research shows that the mother’s emotional adjustment affects her well-being as well as that of the baby and the family. Footnote 51 Mothers may experience a range of emotions postpartum, including baby blues, depression, anxiety disorders, obsessive–compulsive disorders, trauma and stressor-related disorders, and postpartum psychosis.
Compassion and vigilance are the key approaches to effective support for the new mother and family during this period of transition. HCPs will want to attune themselves to the thoughts and experiences of new mothers and their partners in order to help them explore their feelings and emotional health, rather than rely on tasks or checklists. As always, the goal is to empower the mother in her own capacity to adjust and adapt.
The Canadian Task Force on Preventive Health Care guideline Recommendations on Screening for Depression in Adults does not recommend screening of adults by population subgroup, including perinatal and postpartum women, who may be at increased risk of depression. They recommend that clinicians remain alert to the possibility of depression, especially in individuals with characteristics that may increase the risk for depression, and be attentive when there are clinical clues. The Task Force does not have guidelines on screening for other areas of emotional health and mental illness. Footnote 52
Other organizations, such as the Registered Nurses’ Association of Ontario (RNAO), the Ontario Provincial Council for Maternal and Child Health, Perinatal Services BC, and the US Preventative Task Force, do recommend screening pregnant and postpartum women for depression. Footnote 42 Footnote 53 Footnote 54 Footnote 55
It is important that HCPs develop assessment skills to monitor symptoms for mental disorders and stay alert for signs of concern so they can provide appropriate information and support. need to be aware of the various types of responses and sufficiently knowledgeable about emotional health to identify psychiatric disorders in the immediate postpartum phase and beyond (as these disorders do not always present in early postpartum).
Caesarean Birth
Caesarean births are common—in 2016/17, 28% of all births in Canada were by caesarean births. These rates range from 18.5% to 35.3% across the provinces and territories. Footnote 56 Footnote 57
Mothers and families who have an emergency caesarean birth after a long and difficult labour have special needs. They may be experiencing depression, anxiety, guilt, sense of loss of control, less satisfaction with the birth experience, and loss of self-esteem. Footnote 58 Mothers and families who undergo planned, scheduled caesarean births can use coping mechanisms to prepare for the surgery; women undergoing an unplanned caesarean birth do not have this preparation time. Footnote 32 If a woman has an unplanned caesarean birth but feels respect and compassion and that her caregivers are collaborating with her during her labour, her outcomes will likely be optimized. If a woman has an unanticipated caesarean birth and is not supported, she could develop posttraumatic stress disorder (PTSD). Footnote 51 HCPs are well-positioned to help mothers and their families resolve their feelings about the caesarean birth, and connect families to support and services in the community, if needed.
Women who have a caesarean birth need more care and support in their postpartum recovery and greater support caring for themselves and their babies. They experience higher levels of fatigue, constipation, depression, anemia, headache, difficulty voiding, abnormal bleeding, urinary tract infection, abdominal pain, and vaginal discharge than women who have a spontaneous vaginal birth. Primarily because of pain, mothers may need extra help with breastfeeding, especially during the first few days, and they have increased difficulties caring for their babies due to painful or reduced mobility. Footnote 59 It is vital that women and their partners/families understand what to expect during the recovery period, such as the importance of rest, fluids, support for mobility, and adequate diet for recovery. They also need to plan for support with lifting, driving, and household chores.
The average length of hospital stay is longer for women who have caesarean births than for those who have vaginal births. Family support is imperative after a caesarean birth. Mothers and babies should be cared for as a unit, with her partner, if available, including in the NICU.
During the early postpartum period, care of the newborn usually involves celebrating and rejoicing with the family and respecting and supporting their needs. The care is based on nurturing the developing mother–baby–family relationship and caring for mother and baby as a unit. It includes asking the mother and her partner about their concerns and feelings, observing the baby, and supporting his or her health and well-being.
HCPs will want to ensure that the information and advice they share is clear, consistent and tailored to the mother’s specific needs. By focusing on the expressed concerns of the family, rather than on predetermined teaching lists, HCPs will avoid overwhelming them with information. Opportunities to share information about the health and care of babies, including signs of concern, are maximized by caring for mother, baby, and the family together. The mother or partner should be present any time the newborn is being examined, and then made aware of the findings.
There are no Canadian guidelines on the development of newborn care plans. The National Institute for Health and Care Excellence (NICE) and the American College of Obstetricians and Gynecologists (ACOG) recommend developing a documented, individualized postnatal care plan with the woman, ideally in the antenatal period or as soon as possible after the birth. The plan would list the HCPs involved in her and her baby’s care, including their roles and contact details. NICE and ACOG recommend that parents be offered information and advice to enable them to assess their baby’s general condition, identify signs and symptoms of common health problems in babies, and contact an HCP or emergency service if required. Footnote 24 Footnote 60
For babies born in hospitals or birthing centres, the length of their stay varies from a few hours to about 72 hours. Appropriate postpartum follow-up, including a physical examination by a skilled HCP is essential. This physical examination should include observing feeding. The CPS guideline Facilitating Discharge from Hospital of the Healthy Term Infant provides recommendations for discharge and newborn follow-up. Footnote 45
Midwives carry their own caseload and follow their clients regardless of place of birth. They commonly provide three home visits during the first weeks of life.
Baby-friendly Environment and Exclusive Breastfeeding
Breastfeeding is recognized as the unequalled way to provide optimal nutritional, immunological, and emotional nurturing of infants. Footnote 61 Footnote 62 Footnote 63 Footnote 64 Footnote 65 Consistent with WHO global recommendations, Health Canada recommends exclusive breastfeeding for the first 6 months that is sustained for up to 2 years or longer with appropriate complementary feeding. This is important for the nutrition, immunologic protection, growth, and development of infants and toddlers. Footnote 66
It is also important that hospitals, birthing centres, and community health facilities protect, promote, and support breastfeeding, strive for Baby-Friendly status, and achieve the Ten Steps to Successful Breastfeeding.
Infant Mental Health
Infant and early childhood mental health has been defined as “the infant’s/young child’s capacity to experience, regulate, and express emotions, form close and secure relationships, and explore the environment and learn. Footnote 19 ”
Infants form attachments, learn about social interactions and relationships, take in information from the world around them through their five senses, and as they grow, explore their world. Infant mental health is impacted by a number of factors – biology, genetics, brain development, temperament, the prenatal environment, illness or disability, relationships, attachment, their parents’ mental health, parenting, their environment, the social determinants of health, violence, stress and trauma and resiliency. Footnote 67
The basis for mental health starts early in life. Early experiences, including infants’ relationships with parents and caregivers, affect the architecture of their developing brains. Footnote 68 The infant’s brain is growing very fast – and nurturing and responsive caregiving is the key to supporting healthy brain development. Footnote 67 Disruptions in this process can influence stress regulation, emotional health and immune system development throughout life. Footnote 68 Infants are totally dependent on their parents and other caregivers, and when parents and caregivers are responsive, consistent, and nurturing, and they live in safe and economically secure environments, their infants are more likely to have strong emotional health. Footnote 67 Footnote 68 Footnote 69
Parenting and caregiving affect the infant’s/young child’s brain development and mental health through a number of mechanisms. One is attachment. When infants are nurtured and looked after responsively by their parents and other caregivers, their physical and mental health is affected for life through the formation of strong, positive bonds with adults – or attachment. Babies who are securely attached demonstrate less anxiety and more positive emotion in young childhood and are more capable of forming relationships with peers and adults. Footnote 67 Footnote 69
Consistent, high quality and timely daily routines also shape the baby’s developing regulatory system. The predictability and quality of routines influence the biological rhythms related to waking, eating, eliminating, and sleeping. Footnote 67 Footnote 69
On the other hand, if babies experience persistent, toxic stress, the architecture of their brains is weakened. This can lead to mental health issues and physical, learning and behaviour problems throughout life. While stress is an important part of healthy development, when babies without supportive relationships experience high levels of stress for long periods of time, the result is toxic stress. Footnote 70
If parents (or other caregivers) struggle with depression or problematic substance use, for example, they may have difficulty being responsive to their infants. Footnote 71 Footnote 72 Furthermore, if parents have high levels of stress themselves due to precarious economic, housing, or safety conditions, they may struggle to respond to their infants as needed. Footnote 73 Parents in these situations need particular support.
Optimal growth and development requires a continuum of services for infants, toddlers, and their families, delivered by trained professionals. Early investment can support infant mental health and prevent the need for more expensive interventions down the road. Footnote 67 Developing a strong system of informal and formal services is necessary in order to support parents who are struggling to care for their children. In addition, infants/children who are experiencing abnormal stress need assessment and treatment, along with expert support, before this stress has long-lasting effects. Footnote 73
Breastfeeding supports neurodevelopment. This may be due to the breastmilk nutrients or the mother–baby interaction – or both. Neuroscientific evidence strongly supports that infants be exclusively breastfed for 6 months and that hospitalized preterm infants either be breastfed or receive breast milk. Footnote 74 Footnote 75 Consistent with the WHO global recommendation for public health, Health Canada recommends exclusive breastfeeding for the first 6 months that is sustained for up to 2 years or longer, with appropriate complementary feeding to support nutrition needs, for immunological protection and growth and development of infants and toddlers. Footnote 66 Mothers and their families need breastfeeding information and support to encourage exclusive breastfeeding.
Programs that are offered before, during, and after pregnancy as well as during early childhood, have shown benefits for supporting positive infant and child mental health. Footnote 76 These include home-visiting and other family support strategies.
Nobody’s Perfect is a facilitated, community-based parenting program for parents of children from birth to age 5. The program is designed to meet the needs of parents who are young, single, socially or geographically isolated, or who have low income or limited formal education. Several studies have shown that participants in the Nobody's Perfect parenting program experience increased: Footnote 77
- Confidence in their parenting skills;
- Ability to cope with stress;
- Ability to problem solve;
- Resiliency;
- Self-sufficiency and independence;
- Frequency of positive parent–child interactions;
- Use of positive discipline techniques; and
- Access to peer/social/community support.
While there are no national Canadian guidelines on infant mental health, the CPS position statement Relationships Matter: How Clinicians Can Support Positive Parenting in The Early Years offers advice on how physicians can positively affect family health and well-being, support parents, and connect families with community resources. Footnote 78 The Infant Mental Health Promotion coalition from the Hospital for Sick Children has developed best practice guidelines, Competencies for Practice in the Field of Infant Mental Health . These outline the knowledge and skills needed to provide competent care. Footnote 79 Best Start has ready-to-use workshop resources for service providers, Healthy Baby Healthy Brain , that help parents and expectant parents support their baby’s brain development. Footnote 80
Ophthalmia Neonatorum
Prophylaxis for neonatal gonococcal ophthalmia remains mandatory in some provinces and territories. The CPS states that “erythromycin, the only ophthalmic antibiotic eye ointment currently available for use in newborns, is of questionable efficacy. Footnote 81 ” Furthermore, the Society considers that eye prophylaxis is not effective in preventing chlamydial conjunctivitis, and that applying medication to the eyes of newborns may result in mild eye irritation. Footnote 81 They no longer recommend prophylaxis for ophthalmia neonatorum but recommend screening all pregnant women for gonorrhea and chlamydia infection, with treatment and follow-up of those found to be infected. The CPS suggests that mothers who were not screened should be tested at birth, and babies of mothers with untreated gonococcal infection should receive ceftriaxone. Footnote 81
The Public Health Agency of Canada (PHAC) states that “all pregnant women at risk should be screened at the first prenatal visit or at the time of delivery if not previously screened,” and provides guidance for the management of ophthalmia neonatorum. Footnote 82
Mothers look at their baby’s skin regularly, and HCPs can help them understand transient benign skin conditions such as acrocyanosis, baby acne, cutis marmorata (mottling), milia, erythema toxicum neonatorum, and dermal melanocytosis (Mongolian spots). Footnote 83 Footnote 84
For detailed information on valid and reliable skin assessment tools for babies at risk of impaired skin integrity, refer to the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) guideline Neonatal Skin Care . Footnote 83
It is important to share information about skin creams or barriers with parents. While routine application of skin creams or lotions is not necessary for newborns, petroleum emollients have been demonstrated to prevent dermatitis and skin breakdown without increasing the risk of infection. Footnote 24 Footnote 83 Barrier creams or ointments can be applied to the diaper area if reddening is noted.
Parents of both newborn boys and girls need to be made aware of how to properly clean and care for their child’s genitals. For example, in the case of uncircumcised newborn boys, normal foreskin can remain nonretractile until puberty. Footnote 84
Newborn babies are often bathed for aesthetic and hygiene reasons, as opposed to medical indications (i.e., to prevent transmitting certain infectious diseases). Footnote 83 Footnote 85 Vernix caseosa, the waxy white substance found coating the skin of newborn babies, moisturizes the baby’s skin and prevents bacterial cutaneous infections. It should not be washed off, but should be allowed to dry naturally.
The priorities at birth and in the early postpartum period are skin-to-skin contact, breastfeeding, and promoting attachment; the first bath can be postponed. Some families bathe their baby for the first time at home, when the baby is a few days old. Ultimately, “decisions about the frequency of bathing and time of day should be based on the individual baby’s need and consideration of family values and beliefs of the local culture. Footnote 83 ”
Newborns do not require daily bathing. Encourage parents to wash their baby with a warm wet cloth between baths, and to wipe the baby’s face and hands frequently. Footnote 83 Footnote 84
Umbilical Cord Care
Parents need to be informed about care of the umbilical cord. Natural drying is recommended, that is, putting nothing on the cord. Topical drying agents (including isopropyl alcohol) and antibiotics do not reduce cord separation time or frequency of cord infections, and in some cases, they can harm the newborn. Footnote 24 Footnote 83 Footnote 85 Newborns can be bathed with the umbilical cord intact so long as the cord is dried thoroughly afterwards.
Fold down diapers to provide maximum exposure to air and prevent contamination with stool or urine. If the umbilical cord or stump is soiled with urine or stool, the area should be washed with water and dried. Parents need to recognize that fever (38 °C or higher), redness, swelling, drainage (yellow pus), foul-smelling discharge, and bleeding (more than a few spots on the diaper shirt or sleeper) are abnormal findings that they should report to their HCP. Footnote 83 Footnote 84
Circumcision
Parents require accurate, up-to-date, evidence-based information about circumcision so they can make an informed choice for their baby. There is considerable controversy in medical communities regarding circumcision. In their position paper, the CPS outlines the benefits and risks, and does not recommend routine circumcision. Footnote 86 According to the American Academy of Pediatrics, the health benefits of newborn male circumcision outweigh the risks. However, the health benefits are not great enough to recommend universal newborn circumcision. Footnote 87 The Journal of Medical Ethics has an exclusive edition exploring the medical, religious, and social reasons for and against circumcision. Footnote 88 Religious, cultural, and social factors play an important part in the decision to circumcise male babies – these should be considered and respected.
Early Immunization
In most parts of Canada, routine immunizations are not given to newborns. As of 2014, only New Brunswick, the Northwest Territories, and Nunavut include hepatitis B vaccine as part of the immunization schedule at birth. Footnote 89 PHAC recommends that hepatitis B vaccine be given at months 0, 1, and 6 with at least 4 weeks between the first and second dose, at least 2 months between the second and third dose, and at least 4 months between the first and third dose. Alternatively, it can be given as DTaP-HB-IPV-Hib vaccine, which protects against diphtheria, tetanus, pertussis (whooping cough), hepatitis B, polio, and Haemophilus influenzae type b, with the first dose at 2 months of age. Footnote 89
It is recommended that a baby whose mother has tested positive for the hepatitis B surface antigen (HBsAg) receive hepatitis B immunoglobulin and a vaccine within 12 hours of birth. If the mother’s hepatitis B status is not known, and will not be known within 12 hours of birth, HCPs might consider administering the vaccine and the immunoglobulin based on risk factors, erring on the side of caution and administering both when uncertain. If the mother is HBsAg negative, it is reasonable to administer the vaccine to babies who may be at increased risk of exposure to HbsAg-positive household members or those at high risk of being positive. Footnote 89
Parents, grandparents, family, and friends who are in regular contact with a baby should have all their recommended immunizations and these should be up-to-date. Anyone requiring a booster vaccine should get it at least 2 weeks before contact with the baby. Footnote 89 This is particularly important for diphtheria, tetanus, and acellular pertussis adult vaccine, as well as for influenza vaccine.
Cases of vitamin D deficiency still occur in babies in Canada who do not receive vitamin D as a supplement. Footnote 90 Without supplementation, a baby's vitamin D stores will be depleted, particularly if the mother's vitamin D stores are low. Footnote 91 Footnote 92
Nutrition for Healthy Term Infants , a joint statement by Health Canada, CPS, Dietitians of Canada, and the Breastfeeding Committee for Canada, recommends a daily vitamin D supplement of 10 µg (400 IU) for exclusively and partially breastfed babies, from birth to 1 year of age. Children aged 12 to 24 months of age who are breastfed or receive breastmilk should continue to receive this daily vitamin D supplement of 10 µg (400 IU). Footnote 66 Footnote 93 Breastfed babies living in northern latitudes require special attention. In this situation, the CPS suggests that vitamin D supplementation within a range of 400 IU/day to 800 IU/day appears to be safe. Footnote 94
Continuing this supplement is a conservative approach to achieving adequate vitamin D intakes. It also provides a consistent and straightforward public health message. In individual practice, the decision to discontinue the supplement beyond 12 months of age can be informed by a dietary assessment of other contributors of vitamin D, such as cow milk. Footnote 93
Newborn Screening
Newborn screening has been one of the most successful public health programs of this century. It has achieved the goal of detecting hereditary disorders that can result in death or severe long-term disability if not identified prior to the onset of signs. Footnote 95
Current Canadian standards are set at the provincial and territorial level, resulting in variations in the number of screening tests performed in the general categories of endocrine disorders, hemoglobinopathies, fatty acid, amino acid and organic acid disorders, cystic fibrosis, galactosemia, and other disorders. Footnote 96 HCPs would be expected to discuss screening the newborn with parents before and soon after the birth, emphasizing that this is a routine part of their baby’s care that can prevent serious health problems. The newborn screening blood specimen card is completed between 1 and 7 days of age — and ideally between 2 and 3 days of age. If testing is conducted earlier, before 24 hours, repeat the test within 5 days. In Quebec, in addition to blood sampling, a urine sample is obtained at 3 weeks for screening of a number of hereditary conditions.
According to the CPS, all newborns should be screened for hyperbilirubinemia, using a predictive nomogram. The Society recommends measuring bilirubin at the same time as having the metabolic screening test, unless it is required earlier, or at discharge or within 72 hours of birth, whichever comes earlier. This is particularly important if babies go home early, since bilirubin levels will peak at home. Footnote 97
The incidence of critical congenital heart disease (CCHD) in Canada is 3/1000 live births. CCHD accounts for more deaths than any other congenital malformation. Between 10% and 30% of CCHD diagnoses are not made prior to discharge from hospital although early diagnosis and follow-up are essential first steps in preventing infant mortality and morbidity. Footnote 98 Some centres now perform routine pulse oximetry screening to identify babies with CCHD. Used in conjunction with prenatal ultrasound and a physical examination, pulse oximetry screening is the best approach to detecting CCHD in newborns. Footnote 99 The CPS recommends that pulse oximetry screening be performed between 24 and 36 hours after the birth, using the baby’s right hand and either foot to minimize false-positive results. The Society recommends that newborns with abnormal results undergo a thorough evaluation by the most responsible HCP. If a cardiac diagnosis cannot be excluded, newborns with abnormal results would be referred to a pediatric cardiologist. Footnote 100
Hearing Screening
Hearing loss is not a common disorder in the newborn. Profound hearing loss (>70 dB) occurs in approximately 1 to 3 infants per 1000 live births. Together with moderate loss (>40 dB), the prevalence increases to 6 per 1000. Footnote 101 Universal screening for hearing results in earlier diagnosis and intervention and improved language outcomes for children. Footnote 102 The CPS and Speech-Language and Audiology Canada recommend universal screening for all newborns. Footnote 102 Footnote 103
Speech-Language and Audiology Canada recommends that screening be conducted by 1 month of age, in either a hospital or community-based setting. Any suspected hearing loss should be confirmed by 3 months of age, and an intervention implemented by 6 months of age. Footnote 104 Screening policies, however, vary between provinces, with some offering universal screening and others screening only high-risk populations. Footnote 96
HCPs will want to discuss the hearing tests with parents and explain the rationale, how they are performed, and the implications of test results that show possible hearing loss. It is also important to explain the efficacy of the test and the occurrence and meaning of false positives.
Newborns, both preterm and term, have a hypersensitivity to stimuli and are more prone to pain and the consequences of pain. It is critical that they receive effective pain relief. As newborns cannot verbalize, it is up to their caregivers to assess and alleviate their pain. Always keep the number of painful procedures to a minimum; those that are conducted should be evidence-based.
Some effective pain management strategies have been identified for newborns during bedside procedures. Footnote 105 Breastfeeding and skin-to-skin contact together are effective at reducing pain, and this is the first line of pain reduction for procedures such as injections, heel lancing, or venipuncture. Footnote 106
- Skin-to-skin contact reduces pain responses in preterm and term babies. Footnote 107 Skin-to-skin contact should be started approximately 10 to 15 minutes prior to the procedure. Footnote 105
- Breastfeeding should be started approximately 5 minutes before the procedure. Ensure that the baby achieves an effective latch with sustained sucking and swallowing. Footnote 105 Sweet solutions, including breast milk, have analgesic effects on babies. Footnote 108
Refer to the CPS guideline Prevention and Management of Pain in the Neonate on bedside procedure pain management as well as surgery and major procedures. Footnote 109
An optimal amount of sleep for both babies and parents is a priority for parents. Deciding where a baby sleeps is personal and highly variable. The decision may be based on cultural or personal values or the desire to facilitate breastfeeding. Alternatively, it may reflect socioeconomic realities such as unstable housing or poverty resulting in a lack of resources such as a crib. Footnote 110
It is incumbent on all HCPs to work closely with the families to promote safe sleep for their babies. HCPs and parents should discuss the following modifiable risk factors, which reduce the risk of Sudden Infant Death Syndrome (SIDS): Footnote 111
- Breastfeeding of any duration, which provides a protective effect, with exclusive breastfeeding offering greater protection;
- Placing infants to sleep in a crib, cradle, or bassinet—one that meets current Canadian regulations—in the same room and near the parent or caregiver's bed;
- Providing a smoke-free environment—both before and after the birth; and
- Placing infants on their backs to sleep, for every sleep.
PHAC recognizes SIDS and other infant deaths that occur during sleep as major public health concerns. Footnote 111 According to Statistics Canada, 10 babies aged less than 1 year died from SIDS in 2018. Footnote 112 While it is important to differentiate between SIDS and accidental suffocation and strangulation in bed, the American Academy of Pediatrics notes that many of the modifiable and non-modifiable risk factors for SIDS and other sleep-related infant deaths are similar. Footnote 113
There is some confusion around the meaning of the term “co-sleeping.” Sometimes it refers to sleeping in the same bed and sometimes to sleeping in the same room. Room sharing occurs when the baby and adult caregiver sleep on separate surfaces in the same room—a practice that is recommended. Footnote 111 Footnote 113 Bed sharing, when the baby and caregiver share the same sleep surface, is not recommended by either CPS or PHAC.
Parental fatigue can play a significant role in creating unsafe sleep environments for babies and, infrequently, extreme parental fatigue can contribute to accidental suffocation. Footnote 110 A more likely scenario is that parents become so tired that they are less capable of making evidence-based decisions about sleep for either themselves or their babies. HCPs should take a proactive approach when it comes to discussing sleep strategies with parents.
Some parents may be hesitant to reveal their actual sleeping environments to HCPs for fear of reprimand. However, they should be able to make informed decisions about where they intend to place their baby to sleep. The prenatal period is an opportune time for HCPs to bring up safe sleep practices, to inquire about where the parents plan to place their baby to sleep, and to explore factors such as socioeconomic circumstances, cultural practices, and beliefs that may influence safe sleep decisions. Footnote 114 However, this should not be a one-time event – plan on having multiple discussions with parents on the topic of safe sleep.
Effective care requires a coordinated approach that involves ongoing communication between HCPs, parents, families, and other caregivers. The unique beliefs and needs of each family, and their personal and environmental resources, influence their decisions. Footnote 115
Refer to the CPS, Canadian Foundation for the Study of Infant Deaths, Canadian Institute of Child Health, Health Canada, and PHAC Joint Statement on Safe Sleep for more information. Footnote 111
Growth Monitoring
Monitoring a baby’s growth helps identify health or nutrition problems early enough for corrective action to be effective. Footnote 66 Footnote 93 Measurement of growth over time should be combined with clinical, developmental, and behavioural assessments. The WHO Child Growth Standards are based on the growth of breastfed babies. Footnote 116 Standard growth charts show the gradual change in growth velocity.
Babies who are feeding well typically regain their birth weight by 10 to 14 days, double their weight by about 5 months, triple it by 12 months and quadruple it by 2 years of age. Footnote 117 Babies grow quickly during the first 3 months, gaining 20 to 30 g per day in the first 4 weeks, or an average of 0.6 to 1.4 kg per month. Footnote 118
At-risk or Unwell Babies
Routine monitoring of newborns should include evaluation and documentation of vital signs, weight, and feeding in addition to routine screening practices. HCPs responsible for newborn babies should be trained to identify abnormal findings and initiate interventions such as glucose monitoring, saturation monitoring, and positive pressure ventilation. The ACoRN program trains HCPs in a primary survey of at-risk or unwell babies to identify areas of concern that require attention. Footnote 119
4. Complications Related to the Mother
Transition to parenthood is normally a time of intense emotional adjustment that is compounded by sleep disruptions, fatigue, and anxiety about caring for and parenting a baby. It can also be a period of high risk for the development or recurrence of mental illness in new mothers. During this time, any from the entire spectrum of psychiatric disorders may occur. Concerns about women’s mental health are some of the most prevalent problems of the perinatal period. Psychiatric disorders often begin in pregnancy, but onset may also be late into the first postpartum year.
Women with a history of psychiatric disorders are particularly vulnerable, although new onset disorders can occur in any postpartum woman due to the complex interplay of biological, psychological, and social determinants of mental health. Women who have a traumatic birth experience, or women who have ill and/or hospitalized newborns, may be at increased risk of mental health problems. Footnote 51 Inadequate support during the postpartum period can also contribute to or exacerbate mental health problems.
The onset or worsening of depression, anxiety, or other mental illnesses can have serious, long-lasting effects on the mother’s developing relationship with her baby. Postpartum depression—especially when left untreated, resulting in chronic maternal depression—can lead to social, emotional, and behavioural development problems in children, including issues with conduct, emotion regulation, insecure attachment, and poor cognitive outcomes. The effects also depend on factors such as social and material support. Footnote 120 Identifying postpartum mental illnesses and providing appropriate psychological support and possible psychiatric care is important. At the same time, ensure that other medical conditions, such as anemia or thyroid abnormalities or substance use, are not causing or contributing to the symptoms.
Ideally, an interprofessional team cares for a new mother with postpartum mental illness. This requires integrating and, especially, coordinating care and services where interventions and objectives are chosen with and accepted by the mother. Footnote 121 Since postpartum women can experience mental health problems for a long time (more than a year in some cases), having one person coordinate integrated care can help ensure that the care is consistent and ongoing. If the mother agrees, her partner and family may also be involved in decisions regarding her care.
Supporting Women with Postpartum Mental Illness Footnote 51
Supporting women with postpartum mental illness requires a multifaceted, family-centred approach based on the individual needs and experiences of the woman and her family. Effective treatments for postpartum mental health disorders may require referral to a mental health professional. HCPs can support new mothers and families by:
- Knowing how to differentiate between postpartum depression and other anxiety disorders or mental illnesses, including post-traumatic stress disorder (PTSD);
- Being familiar with risk factors associated with postpartum depression and mental illnesses;
- Being able to identify women at risk of developing postpartum emotional disorders and those in difficulty;
- Recognizing the symptoms of mental disorders, from baby blues to postpartum psychosis;
- Knowing about the range of treatment options available for the various postpartum mood disorders, and providing women and their families ways to access the appropriate resources;
- Helping to debunk the “motherhood equals joy and complete fulfillment” myth; and
- Encouraging women to talk about their negative emotions to do with motherhood.
Postpartum Blues
The most common type of postpartum mood change is the postpartum blues, or baby blues . Estimates of prevalence range dramatically, from 15% to 84%. Footnote 122 The postpartum blues are thought to be an effect of the rapid post-childbirth hormonal drop on the neurotransmitter systems involved in mood disorders. Footnote 123 Footnote 124 Footnote 125 Footnote 126 Footnote 127 The challenges of caring for the baby and interrupted sleep are also likely to contribute to the blues. Footnote 128 Footnote 129
Common symptoms of postpartum blues are low mood, emotional lability, tearfulness, fatigue, and irritability. These symptoms are usually transient, beginning shortly after childbirth and resolving on their own within the first few weeks postpartum. Footnote 123 The transient nature of the symptoms helps distinguish postpartum blues from a major depressive episode. Other features that distinguish postpartum blues from a major depressive episode are the lack of severe symptoms, such as persistent insomnia, thoughts of guilt or worthlessness, or suicidal ideation. The reason some women have postpartum blues, while others develop major depression is unknown, but research suggests that genetic predisposition is a factor. Footnote 130 Footnote 131 Postpartum blues are self-limiting and require no treatment other than reassurance and support. Footnote 132 However, early onset, severe, or prolonged blues is associated with postpartum depression, and requires medical attention. Footnote 133
Postpartum Depression
Postpartum depression can affect a woman at any age or socioeconomic status and from any culture. Biological risk factors may include history of depression or untreated depression in pregnancy, while psychosocial risk factors may include poor social support and stressful life events, including issues related to the health of the baby. Footnote 134 Some women are at a higher risk of postpartum depression such as Indigenous women, younger mothers, sexual minority women, and women who are recent immigrants to Canada.
The Diagnostic and Statistical Manual of Mental Disorders, version 5 (DSM-5) qualifies a major depressive episode with peripartum onset when symptoms start in late pregnancy or within the first 4 weeks postpartum. Footnote 135 However, most clinicians define postpartum depression as depression during the first year postpartum. Footnote 136 Footnote 137 Footnote 138
According to 2019 Canadian data, almost one-quarter (23%) of mothers who recently gave birth reported depressive and anxiety symptoms that might or might not be postpartum depression or anxiety because these were just very general screening scales. Prevalence of such feelings was higher among mothers aged under 25 years (30%) than all other age groups. Of the mothers who had these feelings, 31% had been told by an HCP that they had depression or a mood disorder before pregnancy. Almost one-third (32%) of mothers who had these feelings reported that they received mental health treatment since the birth of their baby—39% had counselling, 38% medication (such as anti-depressants), and 23% counselling plus medication. Footnote 139 Women with bipolar disorder are at particularly high risk of developing a depressive episode postpartum. Footnote 140
Recent Canadian research indicates that First Nations mothers had a 20% increase in the mean scores of depressive symptoms compared to White Caucasian mothers in Canada. Footnote 141 A systematic review of the evidence on the prevalence of postpartum mental health disorders in Indigenous women confirmed this finding. Footnote 142 Chronic life stress and trauma are considered key causes of prenatal and postpartum depression among Indigenous women. This life stress is influenced by racism, sexism, domestic and sexual violence, and intergenerational trauma from residential schools and other legacies of colonization. Footnote 9
Symptoms of a Major Depressive Episode Footnote 135
- Persistently low mood and/or loss of interest
- Accompanying low energy
- Sleep and appetite disturbances
- Negative thinking patterns
- In more severe cases, thoughts of self-harm and suicide.
While the symptoms of postpartum depression are similar to those of a major depressive episode outside of the postpartum year, the negative thoughts and images associated with postpartum depression can focus on feelings of failure as a mother, anxiety about the baby’s health and well-being, and guilt about having difficulty with the transition to parenthood. While perinatal suicide is extremely rare, as many as 20% of women report thoughts of self-harm or suicide. Footnote 143
The Canadian Task Force on Preventive Health Care guideline Recommendations on Screening for Depression in Adults does not recommend screening for depression in perinatal and postpartum women. Footnote 52 However, there are tools that can be used to help detect anxiety and depression in the postpartum period.
Tools to Detect Anxiety and Depression Postpartum
The Edinburgh Postnatal Depression Scale (EPDS) Footnote 144 Footnote 145 Footnote 146
This 10-item depression scale:
- Can be used in clinical care or for women at risk of, or showing, symptoms of postpartum depression;
- Can be used any time postpartum, including at regular maternal or baby checks;
- Has an anxiety subscale (items 3, 4, and 5);
- Asks about self-harm thoughts (item 10).
Women with a score higher than 12 (out of 30) have 10 times the likelihood of being diagnosed with postpartum depression than women with a lower score.
Whooley Questions for Depression Screening Footnote 121
There are two Whooley questions for depression:
- During the past month, have you often been bothered by feeling down, depressed, or hopeless?
- During the past month, have you often been bothered by little interest or pleasure in doing things?
If a woman answers yes to either of these questions, it signifies need for further follow-up to determine whether she has depression.
Refer to the Registered Nurses’ Association of Ontario best practice guidelines for effective interventions when caring for mothers with postpartum depression. Footnote 53
Anxiety and Related Disorders
Anxiety is a primary feature of perinatal depression, with the prevalence of anxiety symptoms ranging from 14% to 20% in the postpartum period. Footnote 147 Footnote 148 Footnote 149 Parents often feel anxious about the welfare of the baby, insecure about their parenting abilities, or worry about being alone. However, women can also have anxiety and related disorders, including generalized anxiety disorder, panic disorder, obsessive–compulsive disorder and PTSD. Footnote 134
The Generalized Anxiety Disorder 2-item (GAD-2) questionnaire is a useful tool for identifying generalized anxiety disorder. Footnote 150 The tool has just 2 questions with four possible answers per question: Footnote 151
Over the last 2 weeks, how often have you been bothered by the following problems? Feeling nervous, anxious or on edge? Not at all/Several days/More than half the days/Nearly every day Not being able to stop or control worrying Not at all/Several days/More than half the days/Nearly every day
New parents are naturally nervous when they are beginning to care for their newborn baby. Generalized anxiety disorder, however, is characterized by excessive worry about anticipated events or activities in a way that is difficult to control or interferes with daily functioning. The anxiety can be clustered worries about finances, appearances, maintenance of household duties, and the well-being of the baby, for example. Footnote 152
Panic disorder, affecting about 1% to 3% of new mothers, may cause significant impairment. It can result in the mother experiencing isolation due to her difficulty in leaving the home or being in groups of people. Footnote 152 Footnote 153
Obsessive–compulsive symptoms occur in 4% to 9% of new mothers. These most often include obsessions about contamination, compulsions about checking and ordering, and in some cases, thoughts about the baby being harmed. Footnote 154 Footnote 155 Footnote 156 Footnote 157 The latter can be distinguished from psychosis because women with obsessive–compulsive symptoms have no intention of harming their child and are significantly distressed by these types of thoughts. Obsessive–compulsive symptoms commonly co-exist with a depressive episode. Footnote 155
Trauma- and stressor-related disorders, including PTSD, affect about 3% of postpartum women and up to 15% of high-risk women. Footnote 158 Important risk factors included a history of psychopathology, current depression, and complications during pregnancy, labor and delivery. While it is rare that a stressful birth experience leads to PTSD, risk factors do include having a birth experience different from what was expected and ineffective communication where HCPs do not listen to the woman. Footnote 158
Severe Postpartum Mental Disorders
Bipolar disorder and schizophrenia.
About 2% of pregnant women have a pre-existing bipolar disorder, and less than 1% have a pre-existing psychotic disorder such as schizophrenia. Footnote 159 Women with severe mental disorders are at particularly high risk of relapse in the postpartum period and usually require special mental health care. They are also at high risk of developing postpartum depression. Footnote 140
Evidence suggests that there is a relationship between bipolar disorder and postpartum psychosis, with the majority of cases thought to be variants of bipolar disorder. The risk of relapse in women with primary psychotic disorders increases during the postpartum period. Footnote 160 Footnote 161 Sometimes postpartum psychosis is preceded by hypomanic or manic symptoms. Footnote 162
Women with severe mental disorders and their families require support from professionals and family/friends as well as appropriate treatment to promote optimal health and parenting.
Postpartum Psychosis
Postpartum psychosis, the most severe postpartum psychiatric disorder, is a medical emergency. Postpartum psychosis occurs in approximately 1 in every 600 postpartum women. Footnote 161 It most often occurs during the first week or the first month postpartum, but it can occur later in the postpartum period or at weaning, although the latter is rare.
The primary symptoms of postpartum psychosis reflect a significant change from the woman’s usual personality, with confusion and clouding of consciousness considered classic symptoms. These symptoms may be accompanied by an inability to distinguish thoughts from reality and delusions about herself, her baby, or others. Footnote 163 Footnote 164
Women with a history of bipolar and psychotic illnesses are at increased risk for postpartum psychosis, particularly if they stopped taking medication during pregnancy or in the early postpartum period. Other risk factors include a family history of psychiatric illness (particularly bipolar affective disorder) and sleep deprivation among women with a previous bipolar mood disorder diagnosis. Footnote 140
Women with postpartum psychosis require urgent psychiatric consultation, pharmacological treatment, ongoing support to facilitate the recovery process, and usually hospitalization. Footnote 165 They should not be left to care for their babies alone until the psychosis has resolved. Assess and support safety of the mother and her baby on an individual basis, as delusions may increase the risk of harm to either or both. Family members should be educated and engaged, and ongoing support provided by professionals, community organizations, and family/friends. Footnote 166 Footnote 167
Women who develop postpartum psychosis are at increased risk for reoccurrence during subsequent pregnancies. Footnote 168 Footnote 169
Late postpartum hemorrhage, also called secondary postpartum hemorrhage, can occur 24 hours to 12 weeks after childbirth. The potential causes of late postpartum hemorrhage include retained fragments of the placenta or membranes, sub-involution of the placental site, uterine infection, and coagulation defects. Treatment involves controlling bleeding with medications such as oxytocin, as well as possible blood replacement or surgical intervention.
As most cases of late postpartum hemorrhage occur after women leave birthing facilities, focus the discharge information on expected changes, what amount of bleeding is normal and what amount of bleeding is not normal, causes for concern, and when to contact an HCP or emergency department. If a mother needs to be re-admitted to hospital for late postpartum hemorrhage, it is very important not to separate the mother and baby and to provide support for breastfeeding.
Endometritis
Endometritis is an infection of the reproductive tract. It can occur at any time from birth to 6 weeks postpartum. Endometritis occurs after 1% to 3% of vaginal births and up to 27% of caesarean births. Footnote 170 Endometritis is limited to the uterine cavity but can spread.
A woman with mild endometritis has discharge that is scant or profuse, bloody, and foul smelling. In more severe situations, she has fever, chills, lower abdominal pain or uterine tenderness, anorexia, lethargy, and rapid pulse. Treatment includes administration of antibiotics and can also include rest, a high fluid intake, analgesia as needed, and administration of oxytocics to keep the uterus contracted. Comfort measures are important to relieve the symptoms. Footnote 170 Footnote 171
Women need to be informed about what to expect with regard to normal lochia and vaginal discharge, and should call their HCP if they develop symptoms of endometritis.
Mastitis is an inflammation of the breast that may involve an infection. It is characterized by localized tenderness, redness, and heat, and systemic symptoms of fever, malaise, and occasionally nausea and vomiting. Footnote 172 Mastitis commonly occurs within the first 6 weeks postpartum, but can occur at any point during lactation. It can start as engorgement, develop into non-infective mastitis, and then become infective mastitis. Footnote 172 While the breast is congested/engorged, the most effective treatment is breast emptying—by an electric pump if necessary—and increased water intake.
Mastitis occurs in 10% of breastfeeding women, but some studies have reported the incidence to be as high as 33%. Footnote 173
Encourage mothers to continue breastfeeding. It is important that mothers know their milk is safe for their baby even if they require antibiotics. Frequent feeding and good positioning and latching, with effective milk flow from breast to baby, are preventive factors for mastitis.
Hypertension affects 6% to 10% of pregnant women, but few studies have reported the incidence of postpartum hypertension. Women who have had chronic hypertension, gestational hypertension, preeclampsia, and eclampsia may have preeclampsia postpartum—and may develop preeclampsia for the first time postpartum. Footnote 174 As such, if a mother has hypertensive disorder of pregnancy (HPD), postpartum monitoring is important.
Refer to the SOGC guideline Diagnosis, Evaluation, and Management of the Hypertensive Disorders of Pregnancy for information on care in the first 6 weeks postpartum and beyond. The Working Group recommends checking blood pressure 3 to 6 days following birth, especially if the woman has had a pregnancy complicated by high blood pressure. Footnote 175
During pregnancy, women may develop conditions such as preeclampsia and gestational diabetes mellitus (GDM) that put them at higher risk of heart disease and stroke. Pregnancy-related stroke can happen at any stage of pregnancy, but the greatest risk is during birth and the first few months postpartum. It is usually the result of a pre-existing blood vessel malformation or eclampsia. Peripartum cardiomyopathy (PPCM) is a rare—and often misdiagnosed—form of cardiomyopathy that occurs in the last month of pregnancy and up to 5 months postpartum.
There are a number of risk factors for peripartum cardiomyopathy: multiple pregnancies, twins, preeclampsia and eclampsia, a history of heart problems, excessive alcohol consumption, smoking, diabetes, obesity, unhealthy diet, and African heritage. Footnote 176
It is important to describe the signs and symptoms of heart disease and stroke to women and their families and explain when to talk to an HCP or seek emergency care.
Approximately 53% to 79% of women experience some form of laceration during vaginal birth—most often in the perineal body and commonly first- and second-degree lacerations. The more severe third- and fourth-degree lacerations that result in obstetrical anal sphincter injuries (OASIS) may occur in up to 11% of women who have vaginal births. Footnote 177 OASIS may result in significant problems, including anal incontinence, rectovaginal fistula, and pain, along with increased risk of postpartum urinary retention.
Women who have lacerations during birth need to be made comfortable and helped to recover—and be supported in their confidence in caring for their baby. Provide information so that the woman understands what happened during the birth and the extent of the laceration/injury. Focus on what can help recuperation and healing; that is, rest, hygiene, prevention of constipation, and pain management, as needed, so that they can be actively involved in caring for their babies. Helpful measures include sitz baths, using the side-lying breastfeeding position, avoiding sitting or standing for long periods of time, and seeking and accepting support from family and friends.
The SOGC recommends that HCPs carefully examine all women for perineal or vaginal tears and that anyone with a tear that is more than superficial has a systematic rectal exam for OASIS. Footnote 178 The SOGC guidelines provide recommendations on prophylactic antibiotic administration, the use of laxatives, as well as analgesics for pain, in the case of OASIS. Footnote 178
Refer women who have OASIS to a physiotherapist skilled in helping with this condition. Footnote 178 Footnote 179 The benefits relate to wound healing as well as rehabilitation to restore local and integrated muscle function following the muscle trauma. Scar management may be required to help the woman have intercourse without fear and pain. These considerations may also be relevant for first- and second-degree tears, although in this case referral to physiotherapy is not always necessary.
Women who have experienced female genital mutilation/cutting (FGM/C), also known as circumcision, need particularly sensitive postpartum care. Learning about the cultural, social, psychological, and physical implications of this centuries-old traditional practice will help HCPs talk to mothers appropriately and provide care that is culturally aware and respectful. The perineal area may be extremely painful due to repeated cutting and laceration throughout life compounded by a recent vaginal birth, making even walking difficult. This all makes caring for their baby more problematic. Footnote 180
Following birth, women need additional advice on perineal hygiene. Perineal infections may occur if culturally acceptable methods of cleanliness are not understood by HCPs. For example, using water may be considered impure on religious grounds. Instead, a diluted antiseptic wash may be used for cleaning after voiding.
HCPs will want to address birth control methods, as choice may be limited for women with FGM/C. They may have been taught that touching their genitals is forbidden, and because the vaginal area is sensitive, the use of diaphragms, cervical caps, and sponges is usually not suitable. The most acceptable and reliable method of birth control for women with FGM/C may be intrauterine contraception (IUC, also known as an intrauterine device or IUD). Hormonal contraceptives, either oral or implanted, are also possible. As for all women, the different contraceptive options should be explained carefully and clearly.
HCPs also need to discuss FGM/C with parents and inform them that performing FGM/C is illegal in Canada.
Diastasis recti abdominis (DRA) is defined as a separation of the two sides of the rectus abdominis muscles. Footnote 181 The onset of DRA occurs during pregnancy and the first weeks following birth. Footnote 182 The literature on the prevalence and risk factors for development of this condition is limited. Footnote 181 Footnote 182
A prospective cohort study of 300 first-time pregnant women found the prevalence of mild DRA to be high both during pregnancy and after childbirth: 33% at 21 weeks gestation; 60% at 6 weeks postpartum; 45% at 6 months postpartum; and 33% at 12 months postpartum. There was no difference in reported lumbopelvic pain in women with and without DRA. Footnote 181 In another prospective study of 84 first-time pregnant women, the prevalence of DRA decreased from 100% at gestational week 35 to 39% at 6 months postpartum. Women with DRA at 6 months postpartum were equally likely to report lumbopelvic pain as women without DRA. Footnote 182
A widening of greater than 2.7 cm at the level of the umbilicus is considered to be pathological diastasis of the rectus abdominis muscle. Footnote 183 It can have negative health consequences for women during pregnancy and the postpartum period and beyond, including altered body mechanics and posture, injury of the lumbar spine and pelvis, and impaired pelvic stability. Footnote 184 Footnote 185
Exercise is a protective factor in the development of DRA. Footnote 186 Exercise may reduce the risk of developing DRA as it helps to maintain tone, strength, and control of the abdominal muscle. In addition, women who exercise during and after pregnancy most likely exercised before pregnancy and have better-conditioned abdominal muscles than women who do not exercise. The type of exercise also affects DRA width and recovery time. Footnote 186
It is important to refer women with DRA to pelvic floor physiotherapy. Footnote 187 Physiotherapy or exercises for diastasis recti should not only address the separation but retrain the pelvic floor muscles. More than 70% of women with rectus diastasis cannot do a pelvic floor contraction and therefore are more likely to experience incontinence, prolapse, and pelvic pain. Footnote 188 Consider as well physiotherapy or exercises that address posture, body mechanics, and restricted tissues that may be causing poor movement. A corset or binder is often recommended for separations of 4 finger widths or more. Neuromuscular electrical stimulation also helps to reduce DRA, and if combined with abdominal exercises, can augment the effects. Footnote 189 Some women may meet the criteria for surgery (abdominoplasty) if they have unresolved symptoms that have not responded to exercise. Footnote 187
It is important to encourage women who have had gestational diabetes mellitus (GDM) to breastfeed immediately after childbirth. Breastfeeding helps to lower the risk of neonatal hypoglycemia. Footnote 190
Women with GDM require information about the associated health risks:
- Between 16% and 30% of women with GDM develop type 2 diabetes by 5 to 10 years postpartum, and some women develop type 1 diabetes. Footnote 191
- Metabolic syndrome is more common in women with GDM. Women should be counselled about lifestyle modifications to prevent diabetes and cardiovascular disease. Lifestyle changes can prevent the onset of type 2 diabetes. Footnote 191
- The recurrence rate of GDM in subsequent pregnancies is about 30% to 84%. Footnote 192
For most women with GDM, diabetes goes away soon after childbirth. However, only 50% of women return for postpartum testing due to time pressures, lack of childcare, lack of awareness of the importance of postpartum screening, the unpleasantness of the test, and other factors. Footnote 191 Footnote 192 Footnote 193 The SOGC guideline Diabetes in Pregnancy recommends that women with GDM be screened with a 75 g oral glucose tolerance test (OGTT) between 6 weeks and 6 months postpartum to detect prediabetes and diabetes. Footnote 194 Women with GDM may benefit from the support of a lactation consultant or specialist in case of delayed onset of breastmilk secretion. Footnote 195 Footnote 196
The Canadian Diabetes Association Clinical Practice Guideline Expert Committee recommends that after childbirth women with pregestational diabetes: Footnote 197
- Breastfeed for the many benefits it offers;
- Be carefully monitored as they have a high risk of hypoglycemia postpartum;
- Use metformin and glyburide, if needed, as they can be used during breastfeeding; and
- Have their triglycerides assessed late postpartum.
In addition, women with type 1 diabetes in pregnancy should be screened for postpartum thyroiditis with a thyroid-stimulating hormone (TSH) test at 6 to 8 weeks postpartum.
Postpartum thyroid dysfunction is common and includes hypothyroidism, hyperthyroidism, and postpartum thyroiditis. It is important to observe postpartum women who have thyroid dysfunction in pregnancy. Women who have thyroid disorders can usually breastfeed. Footnote 117
There are no Canadian national guidelines on thyroid disorders in pregnancy or postpartum. The Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum offers advice on diagnosing and managing thyroid conditions during the postpartum period and breastfeeding. Footnote 198
Symphysis pubic dysfunction (SPD) has been described as a collection of signs and symptoms of discomfort and pain in the pelvic area, including pelvic pain radiating to the upper thighs and perineum. Footnote 199 Footnote 200 Footnote 201 While this term has been used to describe pregnancy-associated pain and instability and dysfunction of the symphysis pubis joint (SPJ) or sacroiliac joint (SIJ), the European Guidelines recommend pelvic girdle pain (PGP) as the accepted umbrella term. Footnote 201 PGP symptoms occur due to pelvic ligament relaxation and increased joint mobility in pregnancy, and can vary from mild discomfort to severely debilitating pain. Footnote 202
About 20% of pregnant women experience PGP. Footnote 201 Footnote 203 Risk factors for developing PGP during pregnancy include a history of previous low back pain and previous trauma to the pelvis. Footnote 201 Prolonged and difficult births, often with larger babies, with the women’s legs widely abducted, and possibly assisted by forceps, can also be contributing factors. The reported incidence of clinically persistent PGP from the postpartum stage to 2 years after childbirth ranges from 5% to 8.5%. Footnote 204
In severe cases, the symphysis pubis may partially or completely rupture. Diastasis of the symphysis pubis (DSP), where the gap in the symphysis pubis increases to more than 10 mm, can only be confirmed by diagnostic imaging. Footnote 205 Footnote 206 DSP can occur during pregnancy, childbirth, or the postpartum period. Footnote 206 Although specific recurrences are difficult to predict, women need to be made aware of the high recurrence rate (68–85%) in subsequent pregnancies. Footnote 207 A small subgroup of patients with PGP can develop chronic pain leading to high disability with resistance to physical interventions. These women should receive multidisciplinary care involving medical and psychological intervention.
There are no Canadian guidelines on diagnosing and managing PGP. Guidelines from Ireland and the United Kingdom are consistent in their message that symptoms of pelvic girdle pain are often mild but can be seriously disabling. Footnote 206 Footnote 208 Women should be asked at every postpartum contact whether they are experiencing pelvic girdle or lower back pain. Footnote 206 Footnote 209 Indications of pain and difficulty with walking after giving birth may indicate pubic symphysis diastasis and should not be discounted as a “minor discomfort of childbearing,” but investigated. Do not discount any level of pain—rather, undertake a careful clinical assessment to determine the extent of the pain and any symphysis pubis dysfunction. Assessments should include determining what occurred during pregnancy and childbirth, and running diagnostics and making timely referrals, including to physiotherapy, to avoid long-term and potentially permanent disability. Footnote 201 Footnote 209
In 2016/2017, 13% of women who gave birth in Canada had an assisted vaginal birth, 9% had a vacuum birth, and 4% had a forceps-assisted birth. Footnote 210 Recent evidence reviews have shown that women who had an assisted vaginal birth were more likely than those who had a spontaneous birth to have at least one health problem during the early postpartum period, for example, painful perineum, constipation, hemorrhoids, breakdown of stitches, and urinary or fecal incontinence. They were also more likely to have a painful perineum at 8 weeks postpartum, regardless of whether they had an episiotomy. Footnote 59 Footnote 177
Forceps-assisted and vacuum-assisted births are associated with an increased risk of injury to the vagina, perineum, and anus. Tears are more severe, which may require prolonged healing. Footnote 211 Women who have a forceps-assisted birth have a significantly greater decrease in intra-anal pressure and a greater incidence of a weak pelvic floor. Footnote 212
It is important to focus on the woman’s comfort during the postpartum period, determining if she has any concerns about perineal comfort or healing, as well as pain, discomfort or stinging, odour, incontinence, or dyspareunia. Footnote 24 The integrity and progress in healing of the perineum needs to be assessed, with pain relief or comfort measures offered and their effectiveness assessed. Women need information on the use of ice packs to decrease swelling, care of the perineum, self-inspection, warm water sitz baths, and Kegel exercises to improve perineal tone. Footnote 41
A significant number of women experience urinary and fecal incontinence following childbirth. The condition is both physically and psychologically challenging, and can influence many aspects of women’s lives and recovery.
Urinary Incontinence
During pregnancy and childbirth, the pelvic floor muscles are stretched and weakened, placing women at risk for the development of urinary incontinence. Footnote 213 While urinary incontinence can happen during pregnancy, stress urinary incontinence results from pelvic floor trauma during vaginal birth, especially the first birth. Although antenatal urinary incontinence, obesity, and significant perineal trauma are risk factors, clinical studies have not identified any single responsible event, suggesting that the problem is multifactoral. Footnote 212 Footnote 213 Footnote 214
Some women have temporary urinary incontinence, but others have long-term problems. Footnote 213 According to the Maternity Experiences Survey, 3.4% of all women who gave birth reported urinary incontinence as “a great deal of a problem” in the first 3 months postpartum. Footnote 215 Women who had vaginal births were more likely to report this problem (4.2%) than women who had caesarean births (1.1%). First-time mothers were also more likely to report this problem (4.0%) than multiparous women (2.9%). Footnote 215
Research indicates that women who had a forceps-assisted birth (with or without an episiotomy) were 10 times more likely to have significant perineal trauma than women who delivered by vacuum extraction without an episiotomy. Moreover, 5 years later, almost half of the women who had assisted vaginal births had some degree of urinary incontinence. Footnote 59
When talking with women about urinary incontinence, focus on prevention, muscle toning techniques, and other interventions. Pelvic floor muscle training can prevent urinary incontinence for up to 6 months after first-time mothers have given birth. Footnote 216 There is also evidence that pelvic floor muscle training is appropriate for women with persistent postpartum urinary incontinence. Footnote 216 The effectiveness might be increased with targeted approaches.
The SOGC recommends Kegel exercises for incontinence with follow-up to assess their effectiveness. Combining any necessary lifestyle changes with bladder training plus pelvic muscle exercises is highly effective. Refer to the SOGC guidelines Conservative Management of Urinary Incontinence . Footnote 217
Urinary Retention
Urinary retention is a sudden inability to spontaneously void the bladder or where a woman passes small amounts of urine but is unable to fully empty her bladder. Footnote 218 Footnote 219 Footnote 220 Symptoms of urinary retention include urinary frequency, voiding small amounts, bladder discomfort or pain, straining to void, reduced sensation to void, incomplete emptying of the bladder and urinary incontinence. Footnote 221
Postpartum voiding dysfunction is defined as failure to pass urine spontaneously within 6 hours of vaginal delivery or the removal of a catheter. Footnote 218 If urinary retention is not detected and managed, it can lead to bladder distention or underactivity and longer-term problems such as incontinence and urinary tract infections. Footnote 222 Footnote 223
The causes of urinary retention are not well understood, but likely mechanical, physiological, and neurological factors are involved. Footnote 220
There are no national Canadian guidelines on postpartum urinary retention, but NICE guidelines recommend that if a woman has not passed urine within 6 hours of childbirth, she has warm baths or showers to assist urination. If these actions are not successful, bladder volume should be assessed and catheterization considered. Footnote 24
Fecal Incontinence
According to the Maternity Experiences Survey, 1.8% of all women who gave birth reported that loss of bowel control was most pronounced in the first 3 months postpartum. Footnote 215 First-time mothers were more likely to report this problem (2.2%) than multiparous women (1.4%). Footnote 215 Anal incontinence after childbirth is more prevalent among women who have had a forceps-assisted birth and laceration of the anal sphincter. Footnote 224 Footnote 225 In addition, women who have anal sphincter tears are more than twice as likely to report postpartum fecal incontinence than women without sphincter tears. Footnote 224 Footnote 225
For women who had an OASIS repair, the SOGC recommends prescribing laxatives and non-steroidal anti-inflammatories and acetaminophen as first-line agents, and a single dose of an intravenous antibiotic. HCPs will want to discuss the degree of injury and arrange for appropriate follow-up. The SOGC also recommends that women with anal incontinence be referred for pelvic floor physiotherapy. Footnote 178
Having to remain in hospital for a prolonged period after childbirth can be extremely stressful for families. Mothers may be distanced from their support circle of friends and family. Concerns regarding contact with and care of other children may be a source of stress. Families may be worrying about the mother’s health and the care of siblings; contact with other children and grandparents; travelling logistics to and from the hospital; and work obligations of partners. It is important that HCPs explore these issues with the family and support them as much as possible. Consider referrals to social services if needed and innovative technology-based programs and resources to help keep families connected. When women are sole-parenting, prolonged stay situations can escalate their stress and anxiety and interfere with their recovery.
A prolonged hospital stay requires compassionate and individualized care. Policies should focus on enabling skin-to-skin contact, supporting breastfeeding, and allowing mothers and babies to be together (rooming-in/mother–baby care). Footnote 23
Mothers who are breastfeeding should have the opportunity to feed frequently and on cue for as long as they want and receive help with breastmilk expression, if needed. Footnote 226 If the baby cannot be given their mother’s breastmilk, pasteurized human donor milk is the next best choice. Footnote 20 Footnote 93 Footnote 227 It is incumbent upon HCPs to consult expert resources to determine the effects on the breastfeeding mother and breastfed baby of any medications the mothers is taking. Only a small number of medications are contraindicated while breastfeeding. Footnote 228
5. Complications Related to the Newborn
According to findings from the ACoRN program, complications related to the newborn fall into eight areas of concern:
- Cardiovascular
- Respiratory
- Neurological
- Gastrointestinal or surgical
- Glucose and electrolytes
- Thermoregulation
Refer to the CPS ACoRN program for guidance on neonatal stabilization, support for multidisciplinary teams, and identifying and caring for babies who are unwell or at risk of becoming unwell in the first few hours or days after childbirth. Footnote 119
If the baby has an infection, supportive care with adequate time to share information is essential. The mother and baby should be considered a unit—with non-separation the goal at all times.
Refer to the CPS guidelines for the diagnosis and treatment of infectious disease in newborns.
With the introduction of guidelines for systematic maternal screening and increased use of intrapartum antibiotics, the incidence of group B streptococcal (GBS) sepsis has decreased from 1.7 cases per 1000 live births in 1993 to 0.22 cases per 1000 live births in 2016. Footnote 229 Footnote 230 Despite this, GBS remains the leading cause of neonatal infection in Canada. In 2012, 48% of the cases of early onset neonatal sepsis were due to GBS, while Escherichia coli accounted for 31%. Footnote 231
Evaluating the risk of sepsis is an important part of the newborn assessment. Prompt treatment prevents the progression to severe disease. Babies at risk for sepsis are those where the mother has maternal GBS colonization in the current pregnancy or GBS bacteriuria; a previous baby with invasive GBS disease; prolonged rupture of membranes (≥18 hours); and maternal fever (temperature ≥38 °C).
The CPS guideline Management of Term Infants at Increased Risk of Early Onset Bacterial Sepsis recommends that any newborn with clinical signs suggestive of sepsis immediately undergo diagnostic evaluation and receive antibiotic therapy. The initial signs of sepsis may be subtle; they include respiratory distress, temperature instability, tachycardia, seizures, hypotonia, lethargy, poor peripheral perfusion, hypotension, and acidosis. Refer to this CPS guideline for diagnosis and management of sepsis.
The care of apparently healthy babies who have risk factors should be individualized. The care will depend on the number of risk factors and whether maternal intrapartum antibiotic prophylaxis for GBS was used. The CPS guideline Management of Term Infants at Increased Risk of Early Onset Bacterial Sepsis has recommendations for various clinical situations and the care of infants who appear healthy but nevertheless have risk factors.
Cardiorespiratory distress in the newborn may occur immediately after childbirth or later in the postpartum period. All HCPs caring for newborns must be able to assess respiratory distress, cyanosis, and perfusion. The CPS recommends that all centres in which babies are born have personnel capable of initiating assisted ventilation. Footnote 232 They also recommend following Neonatal Resuscitation Program guidelines for specific resuscitation procedures immediately after the birth and having a written policy regarding the initial care of a baby with respiratory distress outside of each birthing room in each facility. Footnote 232 Regular simulation sessions or other forms of practice scenarios are useful opportunities for continuing education and maintenance of skills.
Heart murmurs are common in the first few days of life and do not normally indicate a significant problem. In the first 24 hours, murmurs are often indicative of flow through the patent ductus arteriosus and disappear following the closure of the ductus. However, any murmur, even within the first 24 hours, must be assessed in the context of the entire physical examination. If a murmur persists or is symptomatic, a more complete evaluation is recommended. Footnote 233 Footnote 234
The incidence of CCHD in Canada is 3/1,000 live births and accounts for more deaths than any other congenital malformation. Between 10% and 30% of CCHD diagnoses are not made prior to discharge from hospital. Early diagnosis and follow-up are essential first steps in preventing infant mortality and morbidity. Footnote 98 Pulse oximetry screening, used in conjunction with prenatal ultrasound and physical examination, is the best approach to detecting CCHD in newborns. Footnote 99
The definition of hypoglycemia in the newborn is controversial. Multiple reviews have concluded that no specific glucose concentration can be linked to clinical signs or neurological injury. Footnote 235 Approximately 12% to 14% of healthy, appropriate-for-gestational-age (AGA), breastfed, term newborns have blood glucose levels of less than 2.6 mmol/L in the first 72 hours of life. Footnote 236
The CPS does not recommend routine blood glucose monitoring in healthy term babies. Footnote 237 Footnote 238 However, it is important to routinely screen babies at risk for hypoglycemia, including babies of mothers with diabetes (gestational and preconception), preterm babies, and both small-for-gestational-age (SGA) (weight < 10th percentile) and large-for-gestational-age (LGA) babies (weight > 90th percentile). The general recommendation for this population is that glucose levels be maintained at 2.6 mmol/L or higher after the first 2 hours of age.
Blood glucose screening of asymptomatic at-risk babies should begin at 2 hours of age and continue at a frequency and duration that depends upon the specific risk factors and until pre-feeding glucose levels have been consistently documented. Footnote 235 Footnote 238 Babies who are unwell or show signs of hypoglycemia, such as jitteriness, lethargy, poor feeding, apnea, or tachypnea, require immediate testing.
Approaches to the management of hypoglycemia depend upon whether it is asymptomatic or symptomatic. Early and frequent skin-to-skin contact and breastfeeding is encouraged in asymptomatic babies, with supplementation with expressed breast milk the next best approach. A breast milk substitute may be considered, if essential. Footnote 238
Refer to the CPS guideline Screening and Management of Newborns At Risk for Low Blood Glucose for information on diagnosis, monitoring, and management of hypoglycemia. Footnote 238
Selective serotonin reuptake inhibitors (SSRIs) are the antidepressant medications most frequently prescribed for the general population and pregnant women. Footnote 239 When considering prescribing or discontinuing SSRIs, HCPs are expected to weigh the potential harms of untreated depression or anxiety against potential risks to the fetus or newborn. SSRIs as a group have not been found to increase risk of major congenital malformations when used in the first trimester. However, paroxetine use in the first trimester may increase the risk of cardiovascular malformation, and other SSRIs may increase the risk of specific birth defects. Footnote 240
Third trimester use of SSRIs has been linked to a constellation of neonatal signs including prolonged crying, jitteriness, increased tone, tachypnea, cyanosis and feeding difficulty. Footnote 241 These signs occur in 10% to 30% of babies exposed to SSRIs in utero, usually within several hours of birth. The signs are usually mild—sometimes so mild they are difficult to identify—and resolve over several weeks. Footnote 242 Footnote 243 The mother is the best person to settle her baby with skin-to-skin contact, breastfeeding, and holding and comforting – the HCP’s role is to support her in caring for her baby and to provide a calm, quiet environment.
The CPS recommends observing the baby in hospital for 48 hours when SSRIs have been used during the third trimester. Footnote 240 However, since the majority of babies exposed to SSRIs are born healthy, Perinatal Services BC recommends considering discharge after 24 hours for babies who show no adverse signs or symptoms and who meet the following criteria:
- normal vital signs and oxygen saturation levels for the first 24 hours and at discharge;
- a normal physical exam;
- established feeding;
- well-regulating temperature; and
- no signs or symptoms of neonatal abstinence syndrome (NAS). Footnote 244
It is important to inform families about the possible effects of SSRIs on their baby and about strategies to support babies with symptoms. Postpartum use of SSRIs is not a contraindication to breastfeeding. Footnote 240 While information about long-term neurodevelopmental outcomes after prenatal SSRI exposure is largely reassuring, evidence in this area is limited.
Refer to the CPS guideline Selective Serotonin Reuptake Inhibitors in pregnancy and infant outcomes on caring for babies with SSRI exposure. Footnote 240
Small for Gestational Age
Babies who are born small for gestational age (SGA) have a birth weight below the 10th percentile of the age- and gestation-specific birth weight. The rate of SGA births has increased steadily between 2008 and 2014, from 8.2 to 9.1 per 100 singleton live births in Canada. Footnote 245
Intrauterine growth restriction (IUGR) describes genetic or environmental factors preventing a fetus reaching its growth potential. Low birth-weight is defined as weight at birth of less than 2500 grams (5.5 pounds) irrespective of gestational age. Footnote 246
Babies with SGA have physical characteristics (behaviour, alertness, spontaneous activity, and feeding ability) similar to those of normal-sized babies of like gestational age. They may look small and thin because they have decreased subcutaneous fat tissue and muscle mass, but they do not have the complications related to organ system immaturity that preterm babies of similar size have. Any complications are usually a function of the underlying cause of the SGA. Footnote 247
Parents and families of babies who are born SGA are anxious about their baby’s well-being and require supportive care, counselling, and reassurance. Caring for the mother–baby unit, non-separation, and supporting breastfeeding are all essential aspects of care.
There is no consensus on the definition of fetal macrosomia, which some define as birth weight of more than 4000, 4500, or 5000 g, regardless of the baby’s gestational age. Others define macrosomia as a baby above the 90th birth-weight percentile for gestational age of a reference population—also known as large-for-gestational-age (LGA) babies. Footnote 248 The description normal birth weight depends on the population of reference.
In Canada, the LGA birth rate among singleton babies decreased from 11.6 to 10.2 per 100 singleton live births between 2005 and 2014. Footnote 245 Babies born with macrosomia are at higher risk of perinatal mortality (stillbirth and early neonatal, late neonatal, and post-neonatal mortality) and have a higher risk of shoulder dystocia, asphyxia, congenital anomalies, infection, and SIDS. Footnote 248
In addition, these babies are more likely to be born with a lower than normal blood sugar level, have a higher risk of childhood obesity, and a higher risk of metabolic syndrome during childhood. Footnote 249 Babies with macrosomia should be assessed for low blood sugar and jaundice and be encouraged to feed soon after the birth to prevent low blood sugar. Footnote 250
Neonatal opioid withdrawal symptoms are a group of possible symptoms experienced by babies whose mothers used opioids during pregnancy. From 48% to 94% of babies exposed to opioids in utero have opioid withdrawal symptoms. Footnote 251
While their symptoms vary, babies who have been exposed to opioids in utero may feed poorly and have diarrhea and weight loss. They may demonstrate tremors, tight muscle tone, excessive crying, hyperactive Moro reflex (sometimes called the startle reflex), irritability, vomiting and convulsions, hyperthermia and tachypnea. Footnote 252 Footnote 253 If these signs become sufficiently severe, and depending on the drug that the baby was exposed to, the baby may require pharmacotherapy.
The CPS recommends that all babies exposed to opioids be assessed using a scoring system that measures the severity of withdrawal symptoms and helps determine the need for additional monitoring, nursing, medical intervention, or pharmacological therapy.
Refer to the CPS practice point Managing Infants Born to Mothers Who Have Used Opioids During Pregnancy for details. Footnote 253
The CPS also notes that the length of stay in hospital varies depending on exposure to opioids prenatally, severity of withdrawal, symptoms, treatment, and social factors. The Society recommends observing babies for a minimum of 72 to 120 hours, depending on their exposure to opioids. If the treatment threshold is not reached within that time, the baby can be discharged. The key to a successful transition home is to ensure continuity of care by an interprofessional team, with anticipatory planning for when the baby meets criteria for discharge. Footnote 253
It is important that babies be cared for in their mothers’ rooms. Having in place a protocol for rooming-in and use of morphine (if required) for opioid-exposed babies helps to reassure staff about the safety of this treatment modality and supports them in caring jointly for the mothers and their babies. The BC Perinatal Services and British Columbia Centre on Substance Use guideline Treatment of Opioid Use Disorder During Pregnancy Guideline Supplement offers a sample rooming-in protocol for opioid-exposed neonates. Footnote 252
Encourage mothers to hold and cuddle their baby as much as possible, as this helps to settle the baby and minimize withdrawal. In addition, if the mother is relaxed, the baby is more likely to relax. Also encourage breastfeeding, as this can delay the onset and decrease the severity of withdrawal symptoms as well as decrease the need for pharmacological treatment. Footnote 253 Footnote 254 Consider that even babies who do not have in utero exposure to opioids usually take at least 36 to 72 hours to settle until the mother’s breast milk comes in and breastfeeding is established.
If the baby requires pharmacotherapy, the mother and baby may be subject to a prolonged hospital stay. It is important to inform the mother during her pregnancy that she and her baby may need to stay longer at the hospital so that she has a realistic understanding of the early postnatal period and be better prepared for any additional care her baby may require. Note that rooming-in and non-pharmacological care often reduce withdrawal signs to the extent that pharmacotherapy treatment is not required. Footnote 252 Footnote 253
Mothers who used opioids during pregnancy may experience a range of emotions; for example, anxiety over the well-being of their baby, concerns about withdrawal signs the baby is showing, and worries about maintaining custody, or they may be confident and relaxed. It is essential to individualize care to support the mother and other caregivers.
The CPS practice point Managing Infants Born to Mothers Who Have Used Opioids During Pregnancy discusses discharge criteria relating to the newborn and referral to support services and family services be considered. Footnote 253 In the Treatment of Opioid Use Disorder During Pregnancy Guideline Supplement , the BC Perinatal Services and British Columbia Centre on Substance Use advise that maternal opioid use alone is not grounds for the apprehension of a baby by authorities or referral to child protection. Make the decision to report on a case-by-case basis, in consultation with the entire health care team, although HCPs should be aware of their legal obligations in this regard. Footnote 252
Late preterm babies (34 +0 to 36 +6 weeks of gestation) vary widely in physiological maturity. The late preterm baby may have inadequate thermoregulation, immature and weak suck and swallow patterns, incomplete adaptation of certain enzyme systems, and poor immunological and respiratory defence systems. Footnote 255 These factors contribute to increased risk of death and morbidity compared to full-term babies. Common problems are hypoglycemia, hypothermia, respiratory distress, infections, increased risk and delayed onset of hyperbilirubinemia, feeding issues, increased hospital readmission rates, and growth failure. Footnote 256 Early term babies (37 +0 to 38 +6 weeks of gestation) are at increased risk for the same problems as late preterm babies, with increased likelihood of admission to NICU. Footnote 257
An assessment at birth to confirm the baby’s gestational age and ongoing monitoring are important to determine the treatment plan. Delay in adaptation might require admission to NICU, while mature late preterm babies can be cared for in regular postpartum care. In both situations, it is important to avoid separating the mother and baby. Footnote 255
Screen for hypoglycemia and hyperbilirubinemia according to the CPS Screening Guidelines for Newborns at Risk for Low Blood Glucose and Guidelines for Detection, Management and Prevention of Hyperbilirubinemia in Term and Late Preterm Newborn Infants . Continued breastfeeding support is necessary to establish feeding and prevent readmission.
The CPS guideline Safe Discharge of the Late Preterm Infant provides detailed criteria for hospital discharge and post-discharge follow-up. Footnote 255 Some key criteria include stable vital signs for at least 12 hours prior to discharge, 24 hours of successful feeding, and avoidance of mother–baby separation before discharge by providing flexible accommodation arrangements for parents. Arrange for a follow-up appointment within 24 to 48 hours of discharge with a community-based HCP, prior to the baby being discharged home.
Instrumental birth involves use of a vacuum extractor or obstetrical forceps. Trauma is the major complication of instrument-assisted birth in the newborn. Trauma may be caused by head compression and traction on the fetal intracranial structures, face, and scalp or by suboptimal instrument placement. Footnote 258 The most serious sequelae of trauma is intracranial hemorrhage, which occurs in 16 to 17 per 10 000 births. Footnote 259 Footnote 260
The overall risk to the newborn from assisted vaginal birth is low. The risks that could occur include bumps, bruises, or marks on the baby’s head or face that heal in a few days or weeks; cone-shaping of the head, which returns to normal within a day or two; injuries to the baby’s scalp, head, and eye; injuries to the nerves in the arm or face—the baby’s face muscles may droop if the nerves are injured but go back to normal when the nerves heal. Footnote 177 Footnote 211 Subgaleal hemorrhage is a very rare but serious outcome. Footnote 177 Footnote 211 If the baby has any trauma from an assisted birth, it is important that the mother and family understand the cause, the care required, and the anticipated outcome.
Refer to the SOGC Advances in Labour and Risk Management (ALARM) course for assessment, monitoring, and care of the newborn with subgaleal hemorrhage.
Along with the joy of birth and the delight of welcoming a baby into the family, parents whose babies are born with congenital anomalies or rare conditions have special needs and may feel a sense of loss. Many factors influence parents’ experience of having a baby with an anomaly: their personal beliefs, culture, and support network; their HCPs’ knowledge and attitude; how the diagnosis is communicated; the information that they are given about their baby’s diagnosis and what they can expect; and their connection to appropriate services and support groups. Footnote 261
In these situations, base all communication on compassion, using clear and simple terms. Footnote 262 Parents require access to the most current information about their baby’s condition in a form they can understand. Footnote 263 They need to understand the immediate care plan and know what to expect in the future. Footnote 261 They should also be told about the necessary resources available —medical services, clinics, specialists, therapy (e.g., physical, occupational, speech, vision), breastfeeding support, dietitians, mental health services, recreation services, and support groups.
When babies are born with anomalies or rare conditions, a team approach to the family’s care is always required. Parents will often be referred to genetics services to help in the diagnosis of their baby. They could also be referred to genetics counselling if they have concerns about future pregnancies.
HCPs are encouraged to take extra time to communicate with the parents and family—including significant family members such as grandparents and siblings. Show compassion, listen carefully as the parents and family express their concerns and feelings, and communicate in a way that everyone can understand. It is also important to ensure privacy when discussing the baby with the parents or family.
It is critical to remind parents and family (often repeatedly) about what to expect when they are caring for their baby. When parents are first told about their baby’s diagnosis, they are often overwhelmed to the point that they are unable to retain information. A designated HCP should follow up with parents through the postpartum period and to repeat information in subsequent meetings, to assess their ability to cope, and to refer them to appropriate services. Footnote 261
Referrals to peer support can be helpful to provide parents with a shared social identity and contribute to feelings of hope. Footnote 264 Peer support can include face-to-face or online support groups relevant to the baby’s specific condition.
A baby remaining in hospital (especially in the NICU) for an extended period can create a great deal of stress for parents and families. The kind of care that the baby receives, and the approach to care, affects not only the baby’s physical well-being but also parent–baby attachment, feeding, neurodevelopmental outcomes, and the overall health and well-being of the baby, parents, and family. The parents and family may be experiencing extreme emotions such as anxiety or depression, or conflicting feelings such as the joy at the birth of their baby and the fears for the baby’s well-being and their ability to provide care.
NICU environments that facilitate shared decision-making and partnerships between parents and professionals and enable parents to be their baby’s primary caregiver, create a more consistent care for the baby. They also protect the baby from trauma associated with the NICU, such as isolation, stress, and lack of support during painful procedures, and provide parents with the opportunity to develop confidence and skill in caring for their babies. Footnote 30 Footnote 265 Footnote 266
Critical elements of family-centred care include: unrestricted presence of the parents, 24/7; parents and family as primary caregivers for their babies with the support and guidance of HCPs; and open, continuous communication. Footnote 267 The basic principles of family-centred care in this context are the same as all family-centred care—dignity and respect, shared decision-making, choice, information exchange, empowerment, and collaboration. Footnote 267 Footnote 268
Improvements in the baby’s weight gain, decreased parental stress and anxiety, and increased high frequency exclusive breastfeeding at discharge are some of the demonstrated benefits of family-centred care in the NICU. Footnote 269 Footnote 270 Others include decreased length of stay, enhanced attachment between parents and babies, and greater family satisfaction. Footnote 267 Footnote 270 Family involvement is critical to enabling all babies to reach their full physical, cognitive, and psychosocial development—including those babies in the NICU. Footnote 270 Footnote 271
The Family-Integrated Care (FICare) model is an extension of the principles of family-centred care, with parents as true partners in their baby’s care within the NICU. This model was developed by a health care team that included parents whose babies had been in the NICU and follows research in Estonia. Footnote 29 Integrating parents into the care team in FICare goes well beyond merely allowing parents to be present and observing their baby’s care. Footnote 272 Rather, parents provide most of the care for their baby while HCPs guide and counsel parents. Footnote 29 Footnote 30
Single-family NICU rooms are now in use in a few centres in Canada as well as in the USA and Europe. The single-room setting has a number of benefits: it provides optimal environmental support to parents; reduces neonatal sepsis; improves baby weight gain; improves breastfeeding rates; improves control of excessive noise and light; improves staff and parental satisfaction with care; reduces parental and staff stress and anxiety; and costs the same, or possibly less, than standard NICUs. Footnote 269 Footnote 273 Footnote 274 Footnote 275 Footnote 276 Footnote 277 Single-room care has not been associated with any increase in adverse outcomes. Footnote 275
The stressful environment of the NICU may add to the risks facing preterm or sick babies due to their physiological vulnerabilities, negatively impacting their growth, with the brain particularly affected. Developmental care refers to a range of strategies designed to reduce the stresses of the NICU and include control of external stimuli, improved clustering of care activities, and positioning or swaddling of the preterm baby. Footnote 278 Footnote 279 While more research is needed, developmental care interventions has demonstrated benefits to the outcomes of preterm babies. Footnote 279
Some families, including Indigenous families and those living in rural and remote areas, may be far from home and have to travel for the birth or if the mother and child are transferred to another facility after the baby is born. Prolonged hospital stays can be particularly stressful for these parents, as they are away from extended family members, friends, and support networks. They may have other children back home, which can cause additional stress.
Providing Family-Centred Care in the NICU Footnote 29 Footnote 268 Footnote 269 Footnote 270 Footnote 271 Footnote 280 Footnote 281
- Are full partners in decision-making and caregiving and are integrated into the NICU team;
- Have unlimited access to their babies and rooming-in, 24/7;
- Are supported by HCPs in aspects of care, such as prolonged skin-to-skin contact, breastfeeding, and providing developmentally appropriate care so that they become competent in their caregiving;
- Are supported in their baby’s care to minimize their baby’s stress and pain, to safeguard their sleep, and protect their baby’s skin;
- Participate in care planning—in rounds and having access to their baby’s records;
- Receive psychosocial support from the interprofessional team, including psychologists, and peers; and
- Are enabled to express their emotions and fears.
Health care providers: Footnote 29 Footnote 267 Footnote 269 Footnote 270 Footnote 271 Footnote 280 Footnote 281 Footnote 282 Footnote 283
- Provide care based on interprofessional collaboration and partnerships with family and other professional providers;
- Include parents as full partners in decision-making and care;
- Shift their role from skilled provider to one of guidance, supporting parents in their role as primary caregivers, 24/7;
- Focus on promoting baby–parent interactions, stressing the critical importance of parents’ presence and rooming-in, and assuring them of unlimited 24-hour information and access to their baby;
- Support parents in a compassionate, respectful way, recognizing their individual needs;
- Support parents in skin-to-skin contact with their babies;
- Support mothers in breastfeeding and feeding their babies breast milk;
- Communicate with families openly and honestly, and spend time listening to the families’ experiences, fears, and concerns;
- Communicate warmly, regularly, in an understandable fashion, and in a culturally appropriate and safe manner;
- Share information between themselves and with parents;
- Are aware of the possibility of posttraumatic stress disorder (PTSD), and screen for depression; and
- Are supported by system leadership who are committed to an integrated team approach to the needs of babies, families, and staff.
Policies: Footnote 29 Footnote 267 Footnote 269 Footnote 270 Footnote 271 Footnote 280 Footnote 284
- Are supported by a clear vision;
- Have full leadership and administrative support;
- Stipulate unlimited access and preferably rooming-in 24/7 and information for parents;
- Stipulate that parents are integral members of the care team, not visitors, and are their babies’ primary caregivers, sharing in decision-making;
- Create opportunities for the participation of parents in support systems;
- Stipulate that HCPs communicate regularly with parents and provide mechanisms to do so;
- Ensure adequate staffing for the model of care in the unit;
- Support ongoing professional development for NICU staff;
- Actively involve parent partners and advocates in the development and monitoring of policies to inform quality improvement, and develop systems to accommodate this; and
- Support early and frequent breastfeeding or breast milk expression, meetings with lactation consultants and adequate follow-up—with a written policy.
Infrastructure and supports Footnote 267 Footnote 271 Footnote 283 Footnote 285 Footnote 286
- The physical setting is supportive of the baby’s well-being and neurodevelopment, i.e., in a single room with enough space and resources to support parents’ presence (e.g., with showers, kitchen, laundry, lounge, etc.) so that the parents can stay in the room with their baby 24/7 (or sleeping rooms available).
- The interprofessional team give the parents psychological and social support, and they have access to peer support.
- The physical environment supports the breastfeeding mother, e.g., provides for intimacy and means of expressing breastmilk, etc.
- Educational materials are available in plain language in a variety of formats (e.g., in writing, video, apps, etc.).
- Mechanisms in place enable parents’ involvement in their baby’s care and inform them of their baby’s well-being, even when they are not present (e.g., by using web cameras).
- Preparation for the transition home begins at the baby’s admission to the NICU, by providing information on the criteria for discharge and baby care, supporting parents to care for their baby, assessing the parents’ social supports, and providing referrals to appropriate services.
- Care planning for the transition to home includes coordination of health and social care plans with any applicable community services, which may require multi-agency collaboration.
6. Late Postpartum
New parents have many different emotions after the birth of their baby. They may feel full of joy and wonder, anxious, overwhelmed, worried and tired. Having a baby brings a myriad of changes—and is very demanding. It takes months or even years to adapt to these changes. Becoming a parent is a deeply significant personal and social transition that involves a change of identity.
When caring for the new mother and her family, the goal of HCPs is to assist her in this transition and recognize and support her role in caring for her baby and nurturing their interdependent relationship. It is critical to spend time listening to mothers and families and to provide support based on their individual needs and experiences.
Providers should let new mothers know that they have faith in them and their ability to care for themselves and their baby. Providers can also help them listen to their intuitions and learn from their experiences so they become more and more confident in their new role. With time, the mother can discover her strengths and her own way of doing things. Footnote 287
Relationships with a partner and family are also undergoing transition. Communication is the key to nurturing these relationships. Talking about feelings, worries, and happiness during this intense period can help keep couples and/or families close. Footnote 287
Healthy early childhood development includes the physical, social/emotional, and language/cognitive domains. Footnote 288 Many health, social, and justice issues later in life have their roots in early childhood. Parents need the supports of HCPs and community programs to assist them in fostering the optimal growth and development of their baby starting from birth.
Postpartum support in the community should be planned according to a family-centred approach to care, based on women’s experiences and needs, while respecting their diversity in the social and cultural contexts of their postnatal experience. Footnote 289 The woman and her partner and newborn belong at the centre of care, with strategies planned and provided to meet their needs, respecting the woman’s preferences and decisions, while ensuring she is treated with kindness, respect, and dignity. Footnote 29 Footnote 289 Footnote 290
Women, newborns, and families have different points of access to postpartum care in the community. These often involve numerous HCPs (e.g., physicians, nurses, and midwives; lactation consultants and registered dieticians; social workers and psychologists) and community-based providers (e.g., postpartum doulas and maternal child health home visitors). They also seek and receive support from their family members and peers.
Successful postpartum support strategies in the community are holistic and comprehensive, applying an efficient and effective interdisciplinary approach to care. Footnote 289 Women should have multiple choices for the kind of supports that meet their needs. It is critical that women be provided with a first/consistent point of contact (for example, a public health nurse, midwife, or nurse practitioner) for when they need to reach out for support.
Hospitals, health centres, community-based organizations, and public health and primary care providers offer postpartum services in Canada. Some jurisdictions have centres that provide education, support, and programming for new mothers and young families. Various models are used, including phone calls, telephone triage services, clinic visits (drop-in and by appointment), and home visits. Footnote 215 With many providers and many groups providing care, and with a lack of coordination across settings, postpartum care runs the risk of fragmentation. As most women who give birth return home after a very short stay in the hospital or birthing centre, the coordination of support in the community is critical.
Planning postpartum care locally allows for the greatest efficiency and effectiveness. NICE guidelines recommend having a coordinating health care professional for each postpartum case and a documented, individualized care plan developed with the woman. Footnote 24 It is essential that mothers and families know about the specific community supports that are available to them in their area, perhaps in the form of a handout or website that lists the information.
While access to professional postpartum support within the community is essential for positive health outcomes for women, children, and families, social support networks have been identified as one of the key determinants of health. Footnote 291 It is also important that women have access to their own social support networks. Social media provides the opportunity for women to form virtual groups for support and information sharing. They can also access a variety of websites with evidence-based information, such as those of PHAC, provincial/territorial governments, and professional organizations, that can provide answers to questions on self and baby care. HCPs can help women identify the websites or social media sites that may be helpful and those that would be best to avoid because they are neither helpful nor evidence-based.
Optimally, planning for the postpartum period starts during pregnancy. Prenatal education classes may provide a source of postpartum support from other families going through the same experiences. Footnote 292
Appendix B provides descriptions of innovative international and Canadian postpartum care models and guidelines. Refer to Appendix C for an outline of the various methods used to deliver postpartum care in the community.
Continued postpartum support and care needs to be provided according to the principles of family-centred care. It is important to determine and respect the woman’s and family’s views, beliefs, and values. The mother should be fully involved in determining the timing and content of each postpartum contact with HCPs so that the care she receives meets her and her baby’s needs and is flexible. Footnote 24 Footnote 41
At each postpartum encounter, the mother and her partner should have the opportunity to express their feelings and concerns and talk about their physical and emotional well-being, breastfeeding, rest, pain or discomfort and any concerns to do with the baby. These encounters provide HCPs with the opportunity to explore how the mother is coping with her daily experiences and her family and social supports, and to encourage women and their families or partners to talk about any changes in mood, emotional state, and behaviour that are outside of the woman’s normal pattern. Footnote 24 HCPs will want to be aware of and look out for the signs of emotional health problems that occur during the weeks and months following birth.
Professionals have developed a number of methods—written standards of care, care plans, maps or paths, managed care, among others—to ensure that criteria for maternal and newborn health and adjustment are observed during the postpartum period. These criteria, also called indicators or outcomes , include specifics about the mother, the baby, and the family’s social or home support system. While these tools are useful, the focus should always be on supporting the mother and baby’s transition based on their individual needs and experiences.
HCPs are ideally positioned to recognize signs of family violence, including intimate partner violence, as well as child exposure to intimate partner violence and other types of child maltreatment. These forms of violence can negatively impact the health of mother and child, and the effects can persist over time. It is important that providers be equipped to recognize and respond safely to situations involving family violence, and to ensure that their interactions or interventions do not revictimize the mother or child.
According to the Maternity Experiences Survey, about 1 in 10 women who have given birth reported experiencing one or more acts of violence in the past 2 years, most often being pushed, grabbed, or shoved in a way that could have hurt them. Footnote 215 Over half (52%) identified their partner, husband, or boyfriend as the perpetrator of this violence. One-third (31%) experienced the violence during pregnancy, and 16% reported that the violence increased after the birth of the baby, 52% that it decreased, and 32% that it stayed the same. Of those women who experienced abuse, 61% reported discussing or receiving information about what to do if they experienced abuse. Footnote 215
Intimate partner violence has been associated with mental health disorders for women, most commonly depression and anxiety disorders, and PTSD. Other effects on mental health include poor self-esteem, sleep disorders, eating disorders, phobias and panic disorders, substance dependence, antisocial personality disorders, and psychosis. Footnote 293 Intimate partner violence is also associated with postpartum depression. Footnote 294
Child maltreatment includes physical, sexual, and emotional/psychological abuse as well as neglect. Exposure to intimate partner violence is also a form of child maltreatment. Footnote 295 Child maltreatment is a major public health issue associated with a broad range of negative health outcomes across the life course. Approximately one-third of Canadian adults (32%) report experiencing physical abuse, sexual abuse, and/or exposure to intimate partner violence before the age of 16 years. Footnote 296
Provincial/territorial child welfare legislation considers exposing a child to intimate partner violence/family violence a form of maltreatment, and HCPs are required to report it. Footnote 296
Violence in the home has a negative impact on babies, whether they experience it directly, for example, receive an injury while held during physical violence, or indirectly, due to their dependence on their primary caregivers for emotional support. Footnote 297 When the primary caregiver is involved in a stressful event, the child’s main source of comfort is a source of fear and distress. This repeated pattern can result in disorders of attachment, which may contribute to behaviour problems in later childhood. Babies and young children who experience repeated violence in the home have reduced capacity to regulate their emotions and behaviour because of their lack of emotional security. Footnote 297
Adverse Childhood Experiences (ACEs) research has shown that traumatic childhood events such as abuse, neglect, and household dysfunction are linked to an increased likelihood of developing physical, behavioural, and social problems in adulthood. Footnote 298
Canadian and WHO guidance do not recommend universal screening for intimate partner violence. Footnote 299 Footnote 300 HCPs are well-positioned to inquire about intimate partner violence when assessing conditions that may have been caused or complicated by violence. In the context of perinatal care, HCPs should consider asking about intimate partner violence during assessment and subsequently as needed. Footnote 300
Before inquiring about intimate partner violence, certain conditions of safety must be met. Safe responses to an adult’s disclosure follow the LIVES protocol: Listening; Inquiring about needs and concerns; Validating; Enhancing safety; and providing a variety of Supports. Footnote 301 The HCP will want to speak with the postpartum woman separately from her partner and any verbal children, and assess her (and any children’s) risk of immediate danger. After intimate partner violence is disclosed and immediate safety is discussed, assess the need for follow-up, considering what care and support is available, as well as the person’s strengths, needs, priorities, and preferences. Footnote 300
Postpartum nutrition and achieving a healthy weight following a pregnancy can impact maternal and child health both in the short and the long term. The SOGC states that postpartum women can achieve optimal nutrition by eating a variety of high quality foods and following the advice in Canada’s Food Guide .
Breastfeeding women have higher energy needs and should therefore eat a little more food each day than non-breastfeeding women. Canada’s Food Guide recommends regular intake of vegetables, fruit, whole grains, and protein foods. Deficiency of certain nutrients, including thiamin, riboflavin, vitamin B6, vitamin B12, choline, vitamin A, vitamin D, selenium, and iodine, can adversely affect the concentration in breastmilk. Footnote 302 Health Canada recommends that all women who could become pregnant, including breastfeeding women, take a daily multivitamin containing 400 mcg (0.4 mg) of folic acid. Footnote 302
Some women, for example those who live in low income, Indigenous women or women who are newly arrived in Canada or refugees, may be at higher risk of nutritional challenges. Footnote 303 Footnote 304 A lack of access to nutritious food, or to knowledge about nutritious food, can compromise women’s and families’ abilities to eat adequately. It is important that women receive nutritional counselling that is relevant to their specific needs and culture.
Refer to the SOGC guideline Canadian Consensus on Female Nutrition: Adolescence, reproduction, menopause, and beyond for components of the maternal diet that may affect those babies who are breastfeeding. Footnote 305
Postpartum weight
Weight loss during the postpartum period should be gradual. There is little evidence that gradual weight loss affects the volume and quality of breastmilk once lactation is established. Footnote 305 The SOGC emphasizes the need for optimal nutrition to achieve a healthy body weight postpartum. Postpartum visits can be opportunities to check on weight retention/reduction, healthy eating habits, and exercise.
Refer to the SOGC guideline Canadian Consensus on Female Nutrition: Adolescence, reproduction, menopause, and beyond for more information. Footnote 305
HCPs are well positioned to recognize circumstances that are cause for concern; for example, a sudden, rapid weight loss or, conversely, if a woman is living with obesity. The scientific knowledge about obesity and its treatment has led to the recognition that obesity is an illness and not a product of an inadequate lifestyle. It is important to avoid shaming and stigma. Footnote 306
Refer to the SOGC guideline Obesity in Pregnancy for recommendations on the counselling and care of women who have obesity.
Many factors influence a woman’s sexuality during the postpartum period: her culture, her experience before and during pregnancy, her relationship, her physiology, and her emotional and psychological state. Footnote 307 This is compounded by the experience of giving birth, fatigue, the physical recovery from labour and birth, the changes her body is undergoing postpartum, caring for her baby, and perineal pain or discomfort.
Faced with the physiological and emotional changes of becoming new parents, intimacy may be challenging for women and their partners to maintain postpartum, but it remains important for the health of their relationship. Both women and providers often find it difficult to discuss postpartum sexual changes, adjustment, and intimacy. However, sexual concerns are common among women, and they welcome their HCP raising the topic and offering support regarding any concerns that she and her partner may have. Footnote 308 Footnote 309
Low or no sexual desire is very common during the postpartum period. A lesser interest in sexual activity than before or during pregnancy is the norm during the first few months to a year after childbirth. Footnote 310 Footnote 311
Between 22% and 86% of women experience changes in sexual functioning postpartum, especially those who have had an assisted vaginal birth as opposed to a spontaneous vaginal birth or caesarean birth. Footnote 312 A number of studies have linked episiotomy or perineal lacerations and operative vaginal birth with dyspareunia, which can persist for a number of months. Footnote 313 Footnote 314 Women who have had a caesarean birth may also have discomfort with intercourse. Footnote 312 Footnote 315
Refer to the SOGC Female Sexual Health Consensus Clinical Guidelines and Sexual and Reproductive Health Counselling by Health Care Professionals for information on the assessment and sexual health care of postpartum women.
Contraception and Prevention of Sexually Transmitted Infection
Postpartum women need information about contraception and preventing sexually transmitted infections (STIs), and about what methods are compatible with breastfeeding. In this regard, the SOGC recommends the following: Footnote 316 Footnote 317
- Lactational amenorrhea method (LAM) can be used for the first 6 months if the woman’s periods have not returned and the baby is exclusively breastfed on demand day and night and is not fed other foods or liquids. Footnote 318 The woman will need to use another form of birth control once her period returns or the baby is older than 6 months, is no longer exclusively breastfeeding, is sleeping through the night, or has long intervals between breastfeeding. Footnote 318
- Postpartum women may be candidates for an IUC, which can be inserted immediately after delivery. However, women are at a higher risk for uterine perforation during insertion of the IUC in the first postpartum year.
- Hormonal contraceptives can be used by non-breastfeeding women from 3 to 4 weeks after they give birth. Some hormonal birth control methods may decrease milk production, but the progestin-only birth control pill does not appear to interfere with lactation. Currently available combined estrogen–progestin birth control pills do not interfere with the quantity or quality of breast milk once feeding is established.
- Condoms are an effective contraceptive option for breastfeeding and non-breastfeeding women. Condoms also protect both partners from STIs.
Refer to the SOGC guidelines The Canadian Contraception Consensus Guidelines for guidance on the use of contraceptive methods in postpartum and breastfeeding women to prevent pregnancy and STIs.
Immunization is a proven cost-effective public health intervention that prevents significant illness, disability, and death. Footnote 319 Vaccines work best when they are given on time, beginning in infancy. Children are immunized early in life because they are vulnerable to diseases and the consequences can be very serious. The vaccination schedule is designed to protect babies and children before they are exposed to vaccine-preventable diseases.
Periodic outbreaks of illnesses such as measles, which can cause death or disability, can result because not all Canadians are immunized. Footnote 320 PHAC reports that 23% of children have not received the full four recommended doses of the diphtheria, whooping cough (pertussis), and tetanus vaccine by the time they are 2 years old. Footnote 321 In the last 10 years, the number of measles outbreaks has increased in several provinces, with five of the outbreaks involving more than 10 cases. These outbreaks are largely a result of the importation of the virus from other countries, with vulnerable children, including those who are not immunized, contracting the illness. Footnote 322
Some parents may be hesitant or resistant to immunizing their babies. The reasons behind children not being fully immunized are complex and context-specific and often community-specific. A vocal few hold anti-vaccine views; they are not the main reason for the lack of coverage, although the number of vaccine-hesitant parents is growing. Some are complacent, taking vaccination rates and herd immunity for granted; some have doubts about the safety or necessity of vaccines, having been convinced by misinformation about adverse effects; while others do not get their children immunized because of the time and effort it can take to do so or they are concerned about the injections causing pain. Footnote 323
The CPS recommends that HCPs acquire the knowledge and skill to work with parents who are hesitant about immunization. Parents often look to HCPs for answers to their questions about immunization. HCPs will want to share evidence-based information about babies’ vaccinations in a manner that is easy for parents to understand and explore any reasons families may have for not immunizing. Footnote 324 Connecting with parents in order to maintain trust and keep the lines of communication open is critical. Each parent requires different information geared to his or her specific needs.
It is important to understand a parent’s specific concerns and to demonstrate care and compassion for both the child and the family. Telling stories about vaccine-preventable disease cases in Canada can help educate parents. Taking the time to convey information clearly, calmly, and effectively can make the difference whether a child is immunized. Footnote 325
Refer to the CPS practice point Working with vaccine-hesitant parents for more information.
The Canadian Immunization Guide, based on guidance from the National Advisory Committee on Immunization (NACI), provides guidelines for immunization of babies, children, youth, and adults, as well as specific recommendations for postpartum and breastfeeding women. The Canadian Immunization Guide includes the schedule for the following vaccinations for babies and toddlers up to 18 months:
- Diphtheria, tetanus, pertussis, polio
- Haemophilus influenza type b
- Pneumococcal disease
- Meningococcal disease
- Hepatitis B
Although NACI makes recommendations at the national level, provinces and territories determine specific programs and schedules. As such, HCPs need to refer to the immunization schedules of their respective jurisdictions.
It is critical that all siblings, parents, grandparents, other family members, and visitors have all their immunizations up-to-date when a baby comes home. This is particularly important if the baby or mother have underlying medical conditions or vulnerabilities that would increase their risk of communicable diseases like whooping cough and influenza.
The birth of a baby involves many transitions and adaptations for the woman, the baby, and the family. Parents feel many different things following the birth of their baby—joy, wonder and happiness, as well as anxiety, worry and fatigue. These are all normal feelings. Mothers adapt physically and psychologically following birth as they face lack of sleep, physical discomfort or pain and relationship changes. Caring for a baby is demanding, requiring many adaptations for parents. While the postpartum period is a normal, healthy time of life, it is also challenging for families, even as parents get comfortable with their roles.
Providing family-centred maternity and newborn care to women, their partners, and families during the postpartum period is an essential component of the care offered by all institutions, agencies, and programs. It is important that HCPs focus on the individual needs and values of the mothers, partners, newborns, and families they are working with. As women, their partners, and immediate families develop attachment and confidence in caring for their newborn babies, they will also require support from extended family and friends, in addition to providers and community programs.
Clinical Practice Guidelines Relating to Postpartum Health
- Alberta Health Services (PDF 3.54 MB)
- Canadian Paediatric Society
- Manitoba Health, Healthy Living and Seniors
- Ontario - Provincial Council for Maternal and Child Health
- Perinatal Services BC
- Reproductive Care Program of Nova Scotia
- Society of Obstetricians and Gynaecologists of Canada
Breastfeeding
- Agence de la santé et des services sociaux de la Capitale-Nationale - Guide pratique en allaitement pour les médecins
- Baby-Friendly Newfoundland & Labrador – Physician's Toolkit Breastfeeding: Quick Reference Guide (PDF 3.78 MB)
- Best Start – Breastfeeding Guidelines for Consultants - Desk Reference
- Health Canada – Nutrition for Healthy Term Infants
- Public Health Agency of Canada - Protecting, Promoting and Supporting Breastfeeding: A Practical Workbook For Community-based Programs
- Toronto Public Health – Breastfeeding Protocols for Health Care Providers (PDF 9.52 MB)
- Best Start - Giving Birth in a New Land: Strategies for Service Providers Working with Newcomers
- Canadian Nurses Association - Promoting Cultural Competence in Nursing (PDF 1.75 MB)
Environmental Health
- Best Start - Playing it Safe - Service Provider Strategies to Reduce Environmental Risks to Preconception, Prenatal & Child Health - Manual
- Health Canada - Our Health, Our Environment: A Snapshot of Environmental Health in Canada (PDF 3.91 MB)
Healthy Weight/Nutrition/Physical Activity
- Canadian Society for Exercise Physiology - Guidelines
- Health Canada - Canada's Food Guide: Canada's Dietary Guidelines
- Health Canada - Canadian Nutrient File
Indigenous Health
- Anishnawbe Health Toronto - Aboriginal Cultural Safety Initiative
- Best Start - Atuaqsijut: Following the Path Sharing Inuit Specific Ways
- Best Start - Open Hearts Open Minds
- Best Start - Pimotisiwin - A Good Path for Pregnant and Parenting Aboriginal Teens - Report
- Best Start - Supporting the Sacred Journey: From Preconception to Parenting for First Nations Families in Ontario
- Provincial Health Services Authority in British Columbia - Indigenous Cultural Safety Training
- Society of Obstetricians and Gynaecologists of Canada - Aboriginal Sexual Health
Intimate Partner Violence
- VEGA Project
- World Health Organization - Violence Info
- Best Start - Welcoming and Celebrating Sexual Orientation and Gender Diversity in Families, From Preconception to Preschool
- Gay and Lesbian Medical Association - Guidelines for Care of Lesbian, Gay, Bisexual and Transgender Patients
- The Joint Commission - Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care for the Lesbian, Gay, Bisexual, and Transgender (LGBT) Community: A Field Guide
Maternal and Newborn Assessment and Care
- Perinatal Services BC - Newborn & Postpartum Toolkit
- Rourke Baby Record
- The American College of Obstetricians and Gynecologists - ACOG Postpartum Toolkit (PDF 3.49 MB)
Medications
- Centers for Disease Control and Prevention - Treating for Two
- Health Canada - Drug Product Database
- Info-Médicaments en Allaitement et Grossesse
- Merck Manual - Professional Version
- MotherToBaby
Mental Health
- Best Start – Perinatal Mood Disorders: An Interdisciplinary Training Video
- Public Health Ontario - Perinatal Mental Health Toolkit
- Registered Nurses' Association of Ontario - Assessment and Interventions for Perinatal Depression
- Saskatchewan Prevention Institute – Resource Catalogue
Oral Health
- Saskatchewan Prevention Institute - Improving the Oral Health of Pregnant Women and Young Children
Substance Use
- Best Start – Prescription Opioid Use
- Perinatal Services BC & British Columbia Centre on Substance Use - Treatment of Opioid Use Disorder During Pregnancy: Guideline Supplement (PDF 467 KB)
- Portico - Primary Care Addiction Toolkit: Opioids misuse and addiction
- Saskatchewan Prevention Institute – Neonatal Abstinence Syndrome
- Best Start - Tobacco Misuse Resources
- CAN-ADAPTT- Guidelines and resources
- CAN-ADAPTT - Pregnets
- Canadian Public Health Association - Stop Smoking: A Smoking Cessation Resource for Those Who Work with Women
- Portico - Primary Care Addiction Toolkit: Smoking cessation
- Registered Nurses' Association of Ontario - Supporting Pre- and Postnatal Women and Their Families Who Use Tobacco
- Saskatchewan Prevention Institute - Environmental Tobacco Smoke: The risk to unborn babies, pregnant women and children
The Netherlands
The Netherlands has a system for postpartum care provided by kraamverzorgenden — maternity home care assistants. Trained caregivers visit the home of new parents and observe the mother and her baby, offer information in baby care and feeding, and even help in household chores, shopping, and if necessary, cooking. The service is popular and, because of a recent shortage of kraamverzorgenden , the average number of hours of maternity home care assistance over the first 8 days after normal childbirth has decreased from 64 to 44 hours. Footnote 326
As a result of guidelines developed by government, insurance companies, and professional organizations, maternity care in the Netherlands is considered “remarkable for its degree of cooperation between caregivers at different levels and locations in the system. Footnote 326 ” Pregnant women can move freely between care settings and caregivers, including midwives, general practitioners, and specialists.
Most babies are born in hospital in France. When families leave the hospital, they are given the telephone number of the nursery nurse in their area and are encouraged to call with any questions or concerns. Newborn babies are issued with a health record book that contains all their medical information—including vaccinations—up to age 16 years. The health record book is considered an essential document, and it aids the communication process between HCPs and families. Footnote 327
Compulsory medical examinations of children are carried out regularly. The first is within 8 days of birth, another is in month 9 or 10, and the last during month 24 or 25. Mothers and children can access interdisciplinary mother and baby care (“Protection maternelle et infantile”) at local maternal and child health clinics. Clinic staff conduct postnatal checks, provide nutritional and health advice, and can administer vaccinations. Footnote 327
Nova Scotia – Healthy Babies, Healthy Families: Postpartum & Postnatal Guidelines
The Government of Nova Scotia’s Healthy Babies, Healthy Families: Postpartum & Postnatal Guidelines provide guidance for the organization of postpartum services. These guidelines were developed to enhance and support the provision of high quality care to women, their babies, and their families across Nova Scotia in the first 6 weeks postpartum. They contain recommendations that focus on physiological stability, infant feeding or nutrition and growth monitoring, psychosocial/family adjustment, parent–child attachment/parenting, building on capacities and strengths, transition to home and community, family access to community support, healthy lifestyles and environments, collaborative practice, and professional competency. Footnote 328
Ontario – Standards of Postnatal Care
The Standards of Postnatal Care articulate the criteria of postnatal care for mothers and babies in Ontario in immediately postpartum. The Standards identify models, methods, or systems for improving coordination of care along with an evaluation framework to monitor their impact. To support the implementation of the Standards of Postnatal Care , another report was developed: Standards of Postnatal Care for Mothers and Newborns in Ontario (Part II): A focus on implementation and evaluation . This report provides an overview of implementation recommendations to enhance the delivery of postnatal care. It also includes a suggested evaluation framework that identifies priority standards for monitoring across the province. Footnote 42
Ontario – Monarch Centre – Ottawa
The Monarch Centre is a multidisciplinary maternal and newborn health clinic providing evidence-based comprehensive care. Following the birth and discharge from hospital, babies born at the Ottawa Hospital and their mothers can be referred to the Monarch Centre for their first 24- to 48-hour check-up. The registered nurses, board-certified lactation consultants, and family doctors at the Monarch Centre specialize in maternal and newborn care, and provide all the necessary breastfeeding support, bilirubin checks for jaundice, newborn screening and full postpartum check-ups, services and follow-up for mother and baby.
The Monarch Centre coordinates discharge directly with hospital providers to make sure that mother and baby are discharged when ready—and when it is medically appropriate. Upon coordinated discharge from hospital, Monarch supports the transition home for the new family. Footnote 329
British Columbia – The Nurse–Family Partnership
The Nurse–Family Partnership (NFP) is an intensive home-visiting program designed to help young first-time mothers and their children. A public health nurse visits women enrolled in the program throughout their pregnancy and until their child reaches 2 years of age. The goals are to improve children’s health and development while improving mothers’ life situations.
McMaster University in Ontario ran a pilot study of the NFP program, and British Columbia is conducting a randomized controlled trial evaluation. In the USA, the program has demonstrated improved parenting, reduced injuries and poisonings, and improved emotional and language development by babies. The mothers have also been found to have benefitted, with greater participation in the workforce and less reliance on social assistance. Footnote 330 Footnote 331
Drop-In Clinics: Usually staffed by nurses, midwives, and lactation consultants, postpartum clinics are geared to mother/baby drop-ins or scheduled visits. The clinic program can be structured for health assessment, health concerns, breastfeeding support, and advice.
Home Visits: A traditional follow-up component of maternal and newborn care is the home visit by either a nurse or midwife. The length and frequency of visits vary according to the needs of the family and the program specifications. Referrals for home visits are made by the hospital or community liaison staff or by the mother herself; often, they are governed by the “urgency” rating of the assessed need. In some areas, home visiting has been discontinued or replaced with community-based supports that the mother must transit to. Some hospitals have initiated home follow-up by their childbirth staff for mothers in need, as identified by risk criteria or need for additional support. Some home-visiting models use a combination of professional and paraprofessional visitors.
Online: Online resources for postpartum information include social media, websites, and blogs. Online resources enable mothers to engage with other mothers, share experiences, and attain information on caring for themselves and their newborn.
Parenting Courses: As with prenatal classes, some parents benefit from group or individual discussions on parenting during the postpartum period and learn more about their roles as parents through these.
Peer Support: Mother-to-mother support provided in various ways—in person, over the phone, via social media or texts. The supporter is or was in a similar situation to the peer. Some peer-to-peer support deals with specific topics such as breastfeeding or postpartum depression, while others provide general postpartum support.
Phone Lines: Some provinces and communities have initiated phone support and advice for new parents. Parents can ask questions, sometimes day or night, about personal, parenting, and postpartum health concerns. Questions usually relate to breastfeeding, crying, coping at home, and community resources. Phone lines can be connected to general health lines or hospital postpartum wards, or run by public health units or community-based organizations.
Physician/Midwife: Follow-up assessments by the physician, midwife, or other HCP in the community or home. Scheduling/timing of visits depends on general maternal and newborn health; complications of pregnancy, birth, and the postpartum period; and available family/community supports.
Telephone Follow-up: A phone call from public/community health nurses or midwives can ensure that the postpartum plan is in place and working well. Specific outcomes related to feeding and mother and baby well-being can be addressed. The telephone interview may result in a referral to a community agency for service, such as a home visit or other follow-up.
The Canada Prenatal Nutrition Program (CPNP) is a Government of Canada program that provides funding to community groups to help improve the health of pregnant women and new mothers and their babies who face, for example, poverty, teen pregnancy, social and geographical isolation, substance use, or family violence, which put their health at risk. CPNP aims to improve the health of women and their babies by increasing the number of babies born at a healthy weight and promoting and supporting breastfeeding. It ensures culturally sensitive prenatal support for Indigenous women and women who have recently immigrated. The program provides nutrition counselling, prenatal vitamins, food, food coupons and food preparation training, counselling in prenatal health and lifestyle, breastfeeding education and support, education and support on baby care and child development, and referrals to other agencies and services. Footnote 332
The Community Action Program for Children (CAPC) is another Government of Canada program that provides funding to community groups whose focus is the promotion of the healthy development of families (with children from birth to 6 years old) who face challenges that put their health at risk—poverty, teen parenting, social and geographical isolation, substance use, and family violence. The program aims to improve healthy child development by improving parenting skills and parent–child relationships; decreasing social isolation; increasing child self-esteem; and providing child-focused activities. Their programs may include nutritional support and collective kitchens; family resource centres; parenting classes and drop-in groups; child health and development activities; outreach and home-visiting programs; and specialized programs, such as support for mothers dealing with substance use. Footnote 333
The acronym LGBTQ 2 is commonly used to include people who identify their sexual orientation as lesbian, gay, bisexual, queer or questioning, and/or who identify their gender identity as transgender. These guidelines recognize that sexual orientation and gender identity exist along a continuum that may change over time, and that the LGBTQ 2 community is diverse. Footnote 334
Return to footnote * referrer
Perry SE, Hockenberry MJ, Lowdermilk DL, Wilson D, Keenan-Lindsay L, Sams CA. Maternal child nursing care in Canada. 2nd ed. Toronto (ON): Elsevier; 2017.
Return to footnote 1 referrer
Higginbottom GMA, Morgan M, Alexandre M, Chiu Y, Forgeron J, Kocay D, et al. Immigrant women's experiences of maternity-care services in Canada: a systematic review using a narrative synthesis. Syst Rev. 2015;4(13).
Return to footnote 2 referrer
Best Start Resource Centre. Giving birth in a new land: strategies for service providers working with newcomers [Internet]. Toronto (ON): Best Start Resource Centre; 2014 [cited 2020 June 22]. Available from: http://www.beststart.org/resources/rep_health/Newcomer_%20Guide_Final.pdf
Return to footnote 3 referrer
Reading CL, Wein F. Health inequalities and social determinants of Aboriginal peoples' health [Internet]. Prince George (BC): National Collaborating Centre for Aboriginal Health; 2009 [cited 2020 June 22]. Available from: https://www.ccnsa-nccah.ca/docs/determinants/RPT-HealthInequalities-Reading-Wien-EN.pdf
Return to footnote 4 referrer
Kolahdooz F, Launier K, Nader K, Yi KJ, Baker P, McHugh TL, et al. Canadian Indigenous women's perspectives of maternal health and health care services: A systematic review. Divers Equal Health Care. 2016;13(5):334-48.
Return to footnote 5 referrer
Best Start Resource Centre. Planning for change, facilitator guide: Workshop for First Nations women about FASD prevention and skills for change [Internet]. Toronto (ON): Best Start Resource Centre; 2019 [cited 2020 June 22]. Available from: https://resources.beststart.org/wp-content/uploads/2016/01/F19-A.pdf
Return to footnote 6 referrer
Wilson D, de la Ronde S, Brascoupé S, Apale AN, Barney L, Guthrie B, et al. Health professionals working with First Nations, Inuit, and Métis consensus guideline. SOGC clinical practice guideline no. 293. J Obstet Gynaecol Can. 2013;35(6 Suppl 2):S1-4.
Return to footnote 7 referrer
Perinatal Services BC. Doula services [Internet]. Vancouver (BC):PSBC; n.d. [cited 2019 Oct 19]. Available from: http://www.perinatalservicesbc.ca/health-professionals/professional-resources/indigenous-resources/doula-services
Return to footnote 8 referrer
Roy A, Thurston WE, Voices and PHACES Study Team. Depression and mental health in pregnant Aboriginal women: Key results and recommendations from the Voices and PHACES study. Final Report [Internet]. Calgary (AB): University of Calgary: 2015 [cited 2020 June 24]. Available from: https://www.ucalgary.ca/wethurston/files/wethurston/voices-phaces-study-finalreport.pdf
Return to footnote 9 referrer
Jumah NA, Wilson D, Shah R. A Canadian survey of postgraduate education in Aboriginal women's health in obstetrics and gynaecology. J Obstet Gynaecol Can. 2013;35(7):647-53.
Return to footnote 10 referrer
Best Start Resource Centre. Welcoming and celebrating sexual orientation and gender diversity in families [Internet]. Toronto (ON): Best Start Resource Centre; 2013 [cited 2020 June 24]. Available from: https://resources.beststart.org/product/j14e-sexual-orientation-gender-diversity-families-manual/
Return to footnote 11 referrer
Hayman B, Wilkes L, Halcomb EJ, Jackson D. Marginalised mothers: Lesbian women negotiating heteronormative healthcare services. Contemp Nurse. 2013;44(1):120-7.
Return to footnote 12 referrer
Gregg I. The health care experiences of lesbian women becoming mothers. Nurs Womens Health. 2018;22(1):40-50.
Return to footnote 13 referrer
Ross LE. Perinatal mental health in lesbian mothers: A review of potential risk and protective factors. Women Health. 2005;41(3):113-28.
Return to footnote 14 referrer
Maccio EM, PhD, Pangburn JA, MSW. The case for investigating postpartum depression in lesbians and bisexual women. Womens Health Issues. 2011;21(3):187-90.
Return to footnote 15 referrer
Ross LE, Steele L, Goldfinger C, Strike C. Perinatal depressive symptomatology among lesbian and bisexual women. Arch Womens Ment Health. 2007;10(2):53-9.
Return to footnote 16 referrer
Queering Parenthood. Postpartum wellbeing study [Internet]. Toronto (ON): University of Toronto; 2007 [cited 2020 June 23]. Available from: http://queeringparenthood.com/postpartumwellbeingstudy.php
Return to footnote 17 referrer
Renaud MT. We are mothers too: childbearing experiences of lesbian families. J Obstet Gynecol Neonatal Nurs. 2007;36(2):190-9.
Return to footnote 18 referrer
Zero to Three. Laying the foundation for early development: Infant and early childhood mental health [Internet]. Washington (DC): Zero to Three; 2009 [cited 2020 June 24]. Available from:https://www.zerotothree.org/resources/443-laying-the-foundation-for-early-development-infant-and-early-childhood-mental-health
Return to footnote 19 referrer
World Health Organization, UNICEF. Implementation guidance: protecting, promoting, and supporting breastfeeding in facilities providing maternity and newborn services: the revised Baby-friendly Hospital Initiative 2018 [Internet]. Geneva (CH): WHO; 2018 [cited 2020 July 3]. Available from: http://apps.who.int/iris/bitstream/handle/10665/272943/9789241513807-eng.pdf?ua=1
Return to footnote 20 referrer
Harrison D. Newborn pain reduction: Evidence based strategies [Internet]. Ottawa (ON): Champlain Maternal Newborn Regional Program; 2017 [cited 2020 July 3]. Available from: http://www.cmnrp.ca/uploads/documents//KT_Strategy__Newborn_Pain_Management_FINAL_revised_2017_12.pdf
Return to footnote 21 referrer
Moore ER, Bergman N, Anderson GC, Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016(11):CD003519.
Return to footnote 22 referrer
Provincial Council for Maternal and Child Health. Final report: Maternal-Newborn Advisory Committee, Mother Baby Dyad Work Group [Internet]. Toronto (ON): PCMCH; 2011 [cited 2020 July 3]. Available from: https://www.pcmch.on.ca/wp-content/uploads/2015/07/MBDC_Report_2011FEB06.pdf
Return to footnote 23 referrer
National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth [Internet]. London (UK): NICE; 2006 [cited 2020 July 3]. Available from: https://www.nice.org.uk/guidance/cg37
Return to footnote 24 referrer
Dumas L, Widström A. Skin2Skin infographic FAQ's for health care providers [Internet]. Ottawa (ON): Leeds, Grenville & Lanark District Health Unit; 2015 [cited 2020 July 3]. Available from: http://www.bfiontario.ca/wp-content/uploads/2012/10/Skin-to-skin-Infographic-FAQ-Leeds-Grenville-Lanark-District-Health-Unit.pdf
Return to footnote 25 referrer
Winberg J. Mother and newborn baby: Mutual regulation of physiology and behavior - A selective review. Dev Psychobiol. 2005;47(3):217-29.
Return to footnote 26 referrer
Örtenstrand A, Westrup B, Brostrom EB, Sarman I, Akerstrom S, Brune T, et al. The Stockholm neonatal family centered care study: Effects on length of stay and infant morbidity. Pediatrics. 2010;125(2):e278-85.
Return to footnote 27 referrer
Jefferies AL, Canadian Paediatric Society, Fetus and Newborn Committee. Kangaroo care for the preterm infant and family. Paediatr Child Health. 2012;17(3):141-3.
Return to footnote 28 referrer
Chalmers B. Family-centred perinatal care: improving pregnancy, birth and postpartum care. Cambridge (UK): Cambridge University Press; 2017.
Return to footnote 29 referrer
O'Brien K, Bracht M, Macdonell K, McBride T, Robson K, O'Leary L, et al. A pilot cohort analytic study of family integrated care in a Canadian neonatal intensive care unit. BMC Pregnancy Childbirth. 2013;13(Suppl 1):S12.
Return to footnote 30 referrer
Hung KJ, Berg O. Early skin-to-skin: after cesarean to improve breastfeeding. MCN Am J Matern Child Nurs. 2011;36(5):318-24.
Return to footnote 31 referrer
Phillips C. Family-centered maternity care. 4th ed. St. Louis (MO): Mosby; 2003.
Return to footnote 32 referrer
Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: A systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22(6):999-1012.
Return to footnote 33 referrer
Public Health Agency of Canada. Perinatal health indicators for Canada 2013. Ottawa (ON): PHAC; 2013.
Return to footnote 34 referrer
Leduc D, Senikas V, Lalonde AB, Ballerman C, Biringer A, Delaney M, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. SOGC clinical practice guideline no. 235. J Obstet Gynaecol Can. 2009;31(10):980-93.
Return to footnote 35 referrer
Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75(6):875-82.
Return to footnote 36 referrer
Evensen A, Anderson JM, Fontaine, P. Postpartum hemorrhage: Prevention and treatment. Am Fam Physician. 2017;95(7):442-9.
Return to footnote 37 referrer
Weiner, G. Textbook of Neonatal Resuscitation. 7th ed. Evanston (IL): American Academy of Pediatrics; 2016.
Return to footnote 38 referrer
Canadian Paediatric Society. Neonatal resuscitation program [Internet]. Ottawa (ON): CPS; 2016 [cited 2020 July 6]. Available from: https://www.cps.ca/en/nrp-prn
Return to footnote 39 referrer
Perinatal Services BC. Provincial perinatal guidelines: Standards for neonatal resuscitation [Internet]. Vancouver (BC): PSBC; 2017 [cited 2020 July 3]. Available from: http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/Newborn/NeonatalResuscitationGuideline.pdf
Return to footnote 40 referrer
Perinatal Services BC. Obstetrics guideline 20: Postpartum nursing care pathway [Internet]. Vancouver (BC): PSBC; 2011 [cited 2020 July 3]. Available from: http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/Maternal/PostpartumNursingCarePathway.pdf
Return to footnote 41 referrer
Provincial Council for Maternal and Child Health. Standards of postnatal care for mothers and newborns in Ontario: A focus on implementation and evaluation [Internet]. Toronto (ON): PCMCH; 2018 [cited 2020 July 3]. Available from: https://www.pcmch.on.ca/wp-content/uploads/2018/05/Standards-of-Postnatal-Care-for-Mothers-and-Newborns-in-Ontario-Final-Report-Part-II-2018May16.pdf
Return to footnote 42 referrer
Provincial Council for Maternal and Child Health. Standards of postnatal care for mothers and newborns in Ontario: Birth to one-week postnatal period [Internet]. Toronto (ON): PCMCH; 2018 [cited 2020 July 3]. Available from: https://www.pcmch.on.ca/wp-content/uploads/2018/05/Standards-of-Postnatal-Care-for-Mothers-and-Newborns-in-Ontario-Final-Report-Part-I-2018May16.pdf
Return to footnote 43 referrer
Breastfeeding Committee of Canada. The BFI 10 steps and WHO code outcome indicators for hospital and community health services [Internet]. Drayton Valley (AB): BCC; 2017 [cited 2020 Jul 3]. Available from: http://breastfeedingcanada.ca/documents/Indicators%20-%20complete%20June%202017.pdf
Return to footnote 44 referrer
Lemyre B, Jefferies AL, O'Flaherty P, Canadian Paediatric Society: Fetus and Newborn Committee. Facilitating discharge from hospital of the healthy term infant. Paediatr Child Health. 2018;23(8):515-22.
Return to footnote 45 referrer
Benitz WE, Watterberg KL, Aucott S, Cummings JJ, Eichenwald EC, Goldsmith J, et al. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135(5):948-53.
Return to footnote 46 referrer
Canadian Institute for Health Information. CIHI snapshot: Inpatient hospitalization, surgery, newborn, alternate level of care and childbirth statistics, 2017–2018 [Internet]. Ottawa (ON): CIHI; 2019 [cited 2020 July 3]. Available from: https://www.cihi.ca/sites/default/files/document/dad-hmdb-childbirth-quick-stats-2017-2018-snapshot-en-web.pdf
Return to footnote 47 referrer
Benahmed N, San Miguel L, Devos C, Fairon N, Christiaens W. Vaginal delivery: how does early hospital discharge affect mother and child outcomes? A systematic literature review. BMC Pregnancy Childbirth. 2017;17(1):289.
Return to footnote 48 referrer
Brown S, Small R, Faber B, Krastev A, Davis P. Early postnatal discharge from hospital for healthy mothers and term infants. Cochrane Database Syst Rev. 2002(3):CD002958.
Return to footnote 49 referrer
Canadian Medical Protective Association. Safe care in obstetrics – Maternal postpartum care: When things don't go as planned after delivery [Internet]. Ottawa (ON): CMPA; 2017 [cited 2020 July 3]. Available from: https://www.cmpa-acpm.ca/en/advice-publications/browse-articles/2017/safe-care-in-obstetrics-maternal-postpartum-care-when-things-don-t-go-as-planned-after-delivery
Return to footnote 50 referrer
Association of Women's Health, Obstetric and Neonatal Nurses. Mood and anxiety disorders in pregnant and postpartum women. J Obstet Gynecol Neonatal Nurs. 2015;44(5):687-9.
Return to footnote 51 referrer
Joffres M, Jaramillo A, Dickinson J, Lewin G, Pottie K, Shaw E, et al. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-82.
Return to footnote 52 referrer
Registered Nurses Association of Ontario. Assessment and interventions for perinatal depression [Internet]. Toronto (ON): RNAO; 2018 [cited 2020 July 3]. Available from: https://rnao.ca/bpg/guidelines/assessment-and-interventions-perinatal-depression
Return to footnote 53 referrer
BC Reproductive Mental Health Program, Perinatal Services BC. Best practice guidelines for mental health disorders in the perinatal period [Internet]. Vancouver (BC): PSBC; 2014 [cited 2020 July 3]. Available from: http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/Maternal/MentalHealthDisordersGuideline.pdf
Return to footnote 54 referrer
Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-7.
Return to footnote 55 referrer
Canadian Institute of Health Information. Inpatient hospitalizations, surgeries and newborn indicators, 2016–2017 [Internet]. Ottawa (ON): CIHI; 2018 [cited 2020 July 3]. Available from: https://www.cihi.ca/sites/default/files/document/hospchild-inpatientalosdiagsurg-2016-2017-en.xlsx
Return to footnote 56 referrer
Canadian Institute of Health Information. Quick stats: Childbirth indicators by place of residence [Internet]. Ottawa (ON): CIHI; 2018 [cited 2020 July 3]. Available from: https://apps.cihi.ca/mstrapp/asp/Main.aspx?Server=apmstrextprd_i&project=Quick%20Stats&uid=pce_pub_en&pwd=&evt=2048001&visualizationMode=0&documentID=029DB170438205AEBCC75B8673CCE822
Return to footnote 57 referrer
Chalmers BE, Dzakpasu S. Interventions in labour and birth and satisfaction with care: The Canadian maternity experiences survey findings. J Reprod Infant Psychol. 2015;33(4):374-87.
Return to footnote 58 referrer
Borders N. After the afterbirth: A critical review of postpartum health relative to method of delivery. J Midwifery Womens Health. 2006;51(4):242-8.
Return to footnote 59 referrer
American College of Obstetricians and Gynecologists. ACOG committee opinion umber 736: Optimizing postpartum care. Obstet Gynecol. 2018;131(5):e140-50.
Return to footnote 60 referrer
Kramer M, Aboud F, Mironova E, Vanilovich I, Platt R, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5):578-84.
Return to footnote 61 referrer
Quigley M, Hockley C, Carson C, Kelly Y, Renfrew M, Sacker A. Breastfeeding is associated with improved child cognitive development: a population-based cohort study. J Pediatr. 2012;160(1):25-32.
Return to footnote 62 referrer
Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess. 2007(153):1-186.
Return to footnote 63 referrer
Arenz S, Rückerl R, Koletzko B, Von Kries R. Breast-feeding and childhood obesity-a systematic review. Int J Obes. 2004;28(10):1247-56.
Return to footnote 64 referrer
Hauck F, Thompson J, Tanabe K, Moon R, Vennemann M. Breastfeeding and reduced risk of sudden infant death syndrome: A meta-analysis. Pediatrics. 2011;128(1):103-110.
Return to footnote 65 referrer
Health Canada, Canadian Paediatric Society, Dietitians of Canada, Breastfeeding Committee for Canada. Nutrition for healthy term infants: recommendations from birth to six months [Internet]. Ottawa (ON): HC; 2012 [cited 2020 July 16]. Available from: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/infant-feeding/nutrition-healthy-term-infants-recommendations-birth-six-months.html
Return to footnote 66 referrer
Zero to Three. The basics of infant and early childhood mental health: A briefing paper [Internet]. Washington (DC): Zero to Three; 2017 [cited 2020 July 3]. Available from: https://www.zerotothree.org/resources/1951-the-basics-of-infant-and-early-childhood-mental-health-a-briefing-paper
Return to footnote 67 referrer
Centers for Disease Control and Prevention. Early brain development and health [Internet]. Atlanta (GA): CDC; 2019 [cited 2020 July 6]. Available from: https://www.cdc.gov/ncbddd/childdevelopment/early-brain-development.html
Return to footnote 68 referrer
Center on the Developing Child. The foundations of lifelong health are built in early childhood [Internet]. Cambridge (MA): Harvard University; 2010 [cited 2020 July 16]. Available from: https://developingchild.harvard.edu/wp-content/uploads/2010/05/Foundations-of-Lifelong-Health.pdf
Return to footnote 69 referrer
National Scientific Council on the Developing Child. Children's emotional development is built into the architecture of their brains: working paper 2 [Internet]. Cambridge (MA): Harvard University; 2004 [cited 2020 July 16]. Available from: https://46y5eh11fhgw3ve3ytpwxt9r-wpengine.netdna-ssl.com/wp-content/uploads/2004/04/Childrens-Emotional-Development-Is-Built-into-the-Architecture-of-Their-Brains.pdf
Return to footnote 70 referrer
van IJzendoorn, M. Attachment at an early age (0-5) and its impact on children's development [Internet]. Rotterdam (NL): Encyclopedia on Early Childhood Development; 2019 [cited 2020 July 16]. Available from: http://www.child-encyclopedia.com/sites/default/files/textes-experts/en/567/attachment-at-an-early-age-0-5-and-its-impact-on-childrens-development.pdf
Return to footnote 71 referrer
Saskatchewan Prevention Institute. Infant and early childhood mental health [Internet]. Saskatoon (SK): SPI; 2011 [cited 2020 July 16]. Available from: https://skprevention.ca/resource-catalogue/child-development/infant-and-early-childhood-mental-health/
Return to footnote 72 referrer
Gunnar MR, Herrera A, Hostinar CE. Stress and early brain development [Internet]. Minneapolis (MN): Encyclopedia on Early Childhood Development; 2009 [cited 2020 July 16]. Available from: http://www.child-encyclopedia.com/sites/default/files/textes-experts/en/580/stress-and-early-brain-development.pdf
Return to footnote 73 referrer
Brown Belfort M. The science of breastfeeding and brain development. Breastfeed Med. 2017;12(8):459-61.
Return to footnote 74 referrer
Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(8):977-85.
Return to footnote 75 referrer
National Collaborating Centre for Determinants of Health. Foundations: definitions and concepts to frame population mental health promotion for children and youth [Internet]. Antigonish (NS): National Collaborating Centres for Public Health; 2017 [cited 2020 July 16]. Available from: http://nccph.ca/images/uploads/general/02_Foundations_MentalHealth_NCCPH_2017_EN.pdf?utm_source=newsletter&utm_medium=email&utm_campaign=PMH_02_En
Return to footnote 76 referrer
Public Health Agency of Canada. Nobody's perfect [Internet]. Ottawa (ON): PHAC; 2019 [cited 2020 July 16]. Available from: https://www.canada.ca/en/public-health/services/health-promotion/childhood-adolescence/parent/nobody-perfect.html
Return to footnote 77 referrer
Canadian Paediatric Society. Relationships matter: How clinicians can support positive parenting in the early years [Internet]. Ottawa (ON): CPS; 2019 [cited 2020 July 16]. Available from: https://www.cps.ca/en/documents/position/positive-parenting
Return to footnote 78 referrer
Infant Mental Health Promotion. Best practice guidelines: Competencies for practice in the field of infant mental health [Internet]. Toronto (ON): IMHP; 2011 [cited 2020 July 16]. Available from: https://www.imhpromotion.ca/Resources/Best-Practice-Guidelines/Competencies.aspx
Return to footnote 79 referrer
Best Start Resource Centre. Healthy baby healthy brain [Internet]. Toronto (ON): Best Start Resource Centre; 2019 [cited 2020 July 16]. Available from: https://resources.beststart.org/product/k34e-healthy-baby-healthy-brain-workshop/
Return to footnote 80 referrer
Moore DL, MacDonald NE, Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Paediatr Child Health. 2015;20(2):93-6.
Return to footnote 81 referrer
Public Health Agency of Canada. Section 5-6: Canadian guidelines on sexually transmitted infections – Management and treatment of specific infections – Gonococcal infections. Table 13: ophthalmia neonatorum [Internet]. Ottawa (ON): PHAC; 2013 [cited 2020 July 23]. Available from: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/sexually-transmitted-infections/canadian-guidelines-sexually-transmitted-infections-34.html#toc361210477
Return to footnote 82 referrer
Association of Women's Health, Obstetric and Neonatal Nurses. Neonatal skin care. 3rd ed. Washington (DC): 2013.
Return to footnote 83 referrer
Canadian Paediatric Society. Your baby's skin [Internet]. Ottawa (ON): CPS; 2017 [cited 2020 July 24]. Available from: https://www.caringforkids.cps.ca/handouts/your-babys-skin
Return to footnote 84 referrer
American Academy of Pediatrics, American College of Obstetricians & Gynecologists. Guidelines for perinatal care. 7 th ed. Washington, DC: 2013.
Return to footnote 85 referrer
Sorokan ST, Finlay JC, Jefferies AL, Canadian Paediatric Society. Newborn male circumcision. Paediatr Child Health. 2015;20(6):311-5.
Return to footnote 86 referrer
American Academy of Pediatrics, Task Force on Circumcision. Circumcision policy statement. Pediatrics. 2012;130(3):585-6.
Return to footnote 87 referrer
Foddy B. Medical, religious and social reasons for and against an ancient rite. J Med Ethics. 2013;39(7):415.
Return to footnote 88 referrer
Public Health Agency of Canada. Recommended immunization schedules: Canadian immunization guide [Internet]. Ottawa (ON): PHAC; 2020 [cited 2020 July 24]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-13-recommended-immunization-schedules.html#p1c12a2
Return to footnote 89 referrer
Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177(2):161-6.
Return to footnote 90 referrer
Butte N, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life [Internet]. Geneva (CH): WHO; 2002 [cited 2020 July 24]. Available from: https://apps.who.int/iris/handle/10665/42519
Return to footnote 91 referrer
Salle BL, Delvin EE, Lapillonne A, Bishop NJ, Glorieux FH. Perinatal metabolism of vitamin D. Am J Clin Nutr. 2000;71(5):1317-24S.
Return to footnote 92 referrer
Health Canada, Canadian Paediatric Society, Dietitians of Canada, Breastfeeding Committee for Canada. Nutrition for healthy term infants: recommendations from six to 24 months [Internet]. Ottawa (ON): HC; 2014 [cited 2020 July 24]. Available from: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/infant-feeding/nutrition-healthy-term-infants-recommendations-birth-six-months/6-24-months.html
Return to footnote 93 referrer
Godel JC, Canadian Paediatric Society, First Nations,Inuit and Métis Health Committee. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583-9.
Return to footnote 94 referrer
Adamkin DH. Metabolic screening and postnatal glucose homeostasis in the newborn. Pediatr Clin North Am. 2015;62(2):385-409.
Return to footnote 95 referrer
Canadian Organization for Rare Disorders. Newborn screening in Canada status report [Internet]. Toronto (ON): CORD; 2015 [cited 2020 July 24]. Available from: https://www.raredisorders.ca/content/uploads/Canada-NBS-status-updated-Sept.-3-2015.pdf
Return to footnote 96 referrer
Barrington K, Sankaran K, Canadian Paediatric Society, Fetus and Newborn Committee. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr Child Health. 2007;12(Suppl B):1-12B.
Return to footnote 97 referrer
Newborn Screening Ontario. Critical congenital heart disease (CCHD) [Internet]. Ottawa (ON): NSO; 2020 [cited 2020 July 24]. Available from: https://www.newbornscreening.on.ca/en/disease/critical-congenital-heart-disease-cchd
Return to footnote 98 referrer
Newborn Screening Ontario. Critical congenital heart disease pulse oximetry screening protocol [Internet]. Ottawa (ON): NSO; 2018 [cited 2020 July 24]. Available from: https://www.newbornscreening.on.ca/sites/default/files/nso_cchd_protocol_v1.1.pdf
Return to footnote 99 referrer
Narvey M, Wong KK, Fournier A, Canadian Paediatric Society, Fetus and Newborn Committee. Pulse oximetry screening in newborns to enhance detection of critical congenital heart disease. Paediatr Child Health. 2017;22(8):494-8.
Return to footnote 100 referrer
Bagatto M, Scollie SD, Hyde M, Seewald R. Protocol for the provision of amplification within the Ontario infant hearing program. Int J Audiol. 2010;49(1):S70-9.
Return to footnote 101 referrer
Patel H, Feldman M. Universal newborn hearing screening. Paediatr Child Health. 2011;16(5):301-5.
Return to footnote 102 referrer
Eskander A, Papsin BC. Screening infants for hearing impairment in Canada. CMAJ. 2014;186(14):1048-9.
Return to footnote 103 referrer
Speech-Language and Audiology Canada. SAC position paper on universal newborn hearing screening in Canada [Internet]. Ottawa (ON): SAC; 2014 [cited 2020 July 24]. Available from: https://www.sac-oac.ca/sites/default/files/resources/SAC-OAC-UNHS-PP_EN.pdf
Return to footnote 104 referrer
Ottawa Neonatal Pain Interest Group. Newborn pain management: A practical approach. Self-learning module [Internet]. Ottawa (ON): Champlain Maternal Newborn Regional Program; 2015 [cited 2020 July 24]. Available from: http://www.cmnrp.ca/uploads/documents//Newborn_Pain_SLM_2015_02_19_FINAL.pdf
Return to footnote 105 referrer
Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2012;12:CD004950.
Return to footnote 106 referrer
Johnston C, Campbell-Yeo M, Disher T, Benoit B, Fernandes A, Streiner D, et al. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev 2017;2017(2):CD008435.
Return to footnote 107 referrer
Harrison D, Bueno M, Yamada J, Adams-Webber T, Stevens B. Analgesic effects of sweet-tasting solutions for infants: Current state of equipoise. Pediatrics. 2010;126(5):894-902.
Return to footnote 108 referrer
Barrington KJ, Batto DG, Finley GA, Wallman C, Canadian Paediatric Society, Fetus and Newborn Committee. Prevention and management of pain in the neonate: An update. Paediatr Child Health. 2007;12(2):137-8.
Return to footnote 109 referrer
Leduc D, Côté A, Woods S, Canadian Paediatric Society. Recommendations for safe sleeping environments for infants and children. Paediatr Child Health. 2004;9(9):659-63.
Return to footnote 110 referrer
Public Health Agency of Canada, Canadian Paediatric Society, Canadian Foundation for the Study of Infant Deaths, Canadian Institutes of Child Health, Health Canada. Joint statement on safe sleep: Preventing sudden infant deaths in Canada [Internet]. Ottawa (ON): PHAC; 2011 [cited 2020 July 24]. Available from: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/hp-ps/dca-dea/stages-etapes/childhood-enfance_0-2/sids/pdf/jsss-ecss2011-eng.pdf
Return to footnote 111 referrer
Statistics Canada. Table: 13-10-0395-01 Leading causes of death, infants [Internet]. Ottawa (ON): SC; 2018 [cited 2020 July 24]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039501
Return to footnote 112 referrer
American Academy of Pediatrics, Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: Updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138(5):e20162938.
Return to footnote 113 referrer
Perinatal Services BC. Health promotion guideline 1: Safe sleep environment guidelines for infants 0 to 12 months [Internet]. Vancouver (BC): Perinatal Services BC; 2011 [cited 2020 July 24]. Available from: http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/HealthPromotion/SafeSleepGuideline.pdf
Return to footnote 114 referrer
Registered Nurses' Association of Ontario. Working with families to promote safe sleep for infants 0-12 months of age [Internet]. Toronto (ON): RNAO; 2014 [cited 2020 July 24]. Available from: https://rnao.ca/bpg/guidelines/safe-sleep-practices-infants
Return to footnote 115 referrer
Bloem M. The 2006 WHO child growth standards. BMJ. 2007;334(7596):705-6.
Return to footnote 116 referrer
Wambach K, Riordan J. Breastfeeding and human lactation. 5th ed. Burlington (MA): Jones & Bartlett Learning; 2016.
Return to footnote 117 referrer
World Health Organization. Weight velocity [Internet]. Geneva (CH): WHO; 2011 [cited 2020 July 24]. Available from: https://www.who.int/childgrowth/standards/w_velocity/en/
Return to footnote 118 referrer
Canadian Paediatric Society. About ACoRN [Internet]. Ottawa (ON): CPS; 2019 [cited 2020 July 24]. Available from: https://www.cps.ca/en/acorn
Return to footnote 119 referrer
Stein A, Pearson RM, Goodman SH, Rapa DPhil E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800-19.
Return to footnote 120 referrer
National Institute for Health and Care Excellence. Antenatal and postnatal mental health: clinical management and service guidance [Internet]. London (UK): NICE; 2014 [cited 2020 Jul 24]. Available from: https://www.nice.org.uk/guidance/cg192
Return to footnote 121 referrer
Henshaw C. Mood disturbance in the early puerperium: a review. Arch Womens Ment Health. 2003;6(S2):s33-42.
Return to footnote 122 referrer
Edhborg M. Comparisons of different instruments to measure blues and to predict depressive symptoms 2 months postpartum: a study of new mothers and fathers. Scand J Caring Sci. 2008;22(2):186-95.
Return to footnote 123 referrer
Watanabe M, Wada K, Sakata Y, Aratake Y, Kato N, Ohta H, et al. Maternity blues as predictor of postpartum depression: A prospective cohort study among Japanese women. J Psychosom Obstet Gynaecol. 2008;29(3):206-12.
Return to footnote 124 referrer
Reck C, Stehle E, Reinig K, Mundt C. Maternity blues as a predictor of DSM-IV depression and anxiety disorders in the first three months postpartum. J Affect Disord. 2009;113(1):77-87.
Return to footnote 125 referrer
Doornbos B, Fekkes D, Tanke MAC, de Jonge P, Korf J. Sequential serotonin and noradrenalin associated processes involved in postpartum blues. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(5):1320-5.
Return to footnote 126 referrer
O'Keane V, Lightman S, Patrick K, Marsh M, Papadopoulos AS, Pawlby S, et al. Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum 'blues'. J Neuroendocrinol. 2011;23(11):1149-55.
Return to footnote 127 referrer
M'baïlara K, Swendsen J, Glatigny-Dallay E, Dallay D, Roux D, Sutter AL, et al. Baby blues: characterization and influence of psycho-social factors. Encéphale. 2005;31(3):331.
Return to footnote 128 referrer
March of Dimes. Baby blues after pregnancy [Internet]. Arlington (VA): March of Dimes; 2017 [cited 2020 July 24]. Available from: https://www.marchofdimes.org/pregnancy/baby-blues-after-pregnancy.aspx
Return to footnote 129 referrer
Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, et al. Elevated brain monoamine oxidase a binding in the early postpartum period. Arch Gen Psychiatry. 2010;67(5):468-74.
Return to footnote 130 referrer
Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, Gutierrez-Zotes A, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193(5):383-8.
Return to footnote 131 referrer
Seyfried LS, Marcus SM. Postpartum mood disorders. Int Rev Psychiatry. 2003;15(3):231-42.
Return to footnote 132 referrer
Henshaw C, Foreman D, Cox J. Postnatal blues: A risk factor for postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):267-72.
Return to footnote 133 referrer
Howard LM, Molyneaux E, Dennis C, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384(9956):1775-88.
Return to footnote 134 referrer
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5 th ed. Washington (DC): 2013.
Return to footnote 135 referrer
Austin M. Classification of mental health disorders in the perinatal period: future directions for DSM-V and ICD-11. Arch Womens Ment Health. 2010;13(1):41-4.
Return to footnote 136 referrer
Jones I, Cantwell R. The classification of perinatal mood disorders—suggestions for DSMV and ICD11. Arch Womens Ment Health. 2010;13(1):33-6.
Return to footnote 137 referrer
Wisner KL, Wisner KL, Moses-Kolko EL, Moses-Kolko EL, Sit DKY, Sit DKY. Postpartum depression: a disorder in search of a definition. Arch Womens Ment Health. 2010;13(1):37-40.
Return to footnote 138 referrer
Statistics Canada. Maternal mental health in Canada, 2018/2019. [Internet]. Ottawa (ON): SC; 2019 [cited 2020 July 24]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/190624/dq190624b-eng.htm
Return to footnote 139 referrer
Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disord. 2003;5(2):98-105.
Return to footnote 140 referrer
Daoud N, O'Brien K, O'Campo P, Harney S, Harney E, Bebee K, et al. Postpartum depression prevalence and risk factors among Indigenous, non-Indigenous and immigrant women in Canada. Can J Public Health. 2019;110(4):440-52.
Return to footnote 141 referrer
Black KA, MacDonald I, Chambers T, Ospina MB. Postpartum mental health disorders in Indigenous women: A systematic review and meta-analysis. J Obstet Gynaecol Can. 2019;41(10):1470-78.
Return to footnote 142 referrer
Wisner KL, Sit DKY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490-8.
Return to footnote 143 referrer
Cox J, Holden J, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150(6):782-6.
Return to footnote 144 referrer
Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Ove Samuelsen S. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104(4):243-9.
Return to footnote 145 referrer
Brouwers EPM, van Baar AL, Pop VJM. Does the Edinburgh postnatal depression scale measure anxiety? J Psychosom Res. 2001;51(5):659-63.
Return to footnote 146 referrer
Matthey S, Barnett B, Howie P, Kavanagh DJ. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? J Affect Disord. 2003;74(2):139-47.
Return to footnote 147 referrer
Figueiredo B, Conde A. Anxiety and depression in women and men from early pregnancy to 3-months postpartum. Arch Womens Ment Health. 2011;14(3):247-55.
Return to footnote 148 referrer
Dennis C, Coghlan M, Vigod S. Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory? J Affect Disord. 2013;150(3):1217-20.
Return to footnote 149 referrer
Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24-31.
Return to footnote 150 referrer
eMentalHealth.ca. Screening tool: anxiety disorders (2-question screener [GAD-2]) [Internet]. Ottawa (ON): eMentalHealth.ca; 2019 [cited 2020 July 24]. Available from: https://www.ementalhealth.ca/index.php?m=survey&ID=44
Return to footnote 151 referrer
Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: A systematic review. J Clin Psychiatry. 2006;67(8):1285-98.
Return to footnote 152 referrer
Weisberg RB, Paquette JA. Screening and treatment of anxiety disorders in pregnant and lactating women. Womens Health Issues. 2002;12(1):32-6.
Return to footnote 153 referrer
Uguz F, Akman C, Kaya N, Cilli AS. Postpartum-onset obsessive-compulsive disorder: incidence, clinical features, and related factors. J Clin Psychiatry. 2007;68(1):132-8.
Return to footnote 154 referrer
Zambaldi CF, Cantilino A, Montenegro AC, Paes JA, de Albuquerque TLC, Sougey EB. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-9.
Return to footnote 155 referrer
Uguz F, Gezginc K, Zeytinci IE, Karatayli S, Askin R, Guler O, et al. Obsessive-compulsive disorder in pregnant women during the third trimester of pregnancy. Compr Psychiatry. 2007;48(5):441-5.
Return to footnote 156 referrer
Speisman BB, Storch EA, Abramowitz JS. Postpartum obsessive‐compulsive disorder. J Obstet Gynecol Neonatal Nurs. 2011;40(6):680-90.
Return to footnote 157 referrer
Grekin R, O'Hara MW. Prevalence and risk factors of postpartum posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2014;34(5):389-401.
Return to footnote 158 referrer
Vigod SN, Seeman MV, Ray JG, Anderson GM, Dennis CL, Grigoriadis S, et al. Temporal trends in general and age-specific fertility rates among women with schizophrenia (1996–2009): A population-based study in Ontario, Canada. Schizophr Res. 2012;139(1):169-75.
Return to footnote 159 referrer
Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry. 2003;64(11):1284-92.
Return to footnote 160 referrer
Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. 2006;296(21):2582-9.
Return to footnote 161 referrer
Heron, McGuinness, Blackmore ER, Craddock, Jones. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-53.
Return to footnote 162 referrer
Ganjekar S, Desai G, Chandra PS. A comparative study of psychopathology, symptom severity, and short-term outcome of postpartum and nonpostpartum mania. Bipolar Disord. 2013;15(6):713-8.
Return to footnote 163 referrer
Engqvist I, Nilsson K. Experiences of the first days of postpartum psychosis: an interview study with women and next of kin in Sweden. Issues Ment Health Nurs. 2013;34(2):82-9.
Return to footnote 164 referrer
Heron J, Gilbert N, Dolman C, Shah S, Beare I, Dearden S, et al. Information and support needs during recovery from postpartum psychosis. BJOG. 2012;15(3):155-65.
Return to footnote 165 referrer
Spinelli MG. Maternal infanticide associated with mental illness: prevention and the promise of saved lives. Am J Psychiatry. 2004;161(9):1548-57.
Return to footnote 166 referrer
Doucet S, Letourneau N, Blackmore ER. Support needs of mothers who experience postpartum psychosis and their partners. J Obstet Gynecol Neonatal Nurs. 2012;41(2):236-45.
Return to footnote 167 referrer
Robertson E, Jones I, Haque S, Holder R, Craddock N. Risk of puerperal and non-puerperal recurrence of illness following bipolar affective puerperal (post-partum) psychosis. Br J Psychiatry. 2005;186(3):258-9.
Return to footnote 168 referrer
Blackmore ER, Rubinow DR, O'Connor TG, Liu X, Tang W, Craddock N, et al. Reproductive outcomes and risk of subsequent illness in women diagnosed with postpartum psychosis. Bipolar Disord. 2013;15(4):394-404.
Return to footnote 169 referrer
Mackeen AD, Mackeen AD, Packard RE, Packard RE, Ota E, Ota E, et al. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015(2):CD001067.
Return to footnote 170 referrer
Smith Murray S, Slone McKinney E. Foundations of maternal-newborn & women's health nursing. 7 th ed. Toronto (ON): Elsevier; 2019.
Return to footnote 171 referrer
Lawrence RA, Lawrence RM. Breastfeeding: a guide for the medical professional. 8 th ed. New York (NY): Elsevier; 2015.
Return to footnote 172 referrer
Spencer JP, MD. Management of mastitis in breastfeeding women. Am Fam Physician. 2008;78(6):727-32.
Return to footnote 173 referrer
Bramham K, Nelson-Piercy C, Brown MJ, Chappell LC. Postpartum management of hypertension. BMJ. 2013;346(7897):30-4.
Return to footnote 174 referrer
Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-38.
Return to footnote 175 referrer
Heart and Stroke Foundation of Canada. Women's unique risk factors [Internet]. Ottawa (ON): Heart and Stroke Foundation of Canada; n.d. [cited 2020 July 24]. Available from: https://www.heartandstroke.ca/heart/risk-and-prevention/womens-unique-risk-factors
Return to footnote 176 referrer
American College of Obstetricians and Gynecologists. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynaecol. 2016;128(1):e1-15.
Return to footnote 177 referrer
Harvey M, Pierce M, Walter J, Chou Q, Diamond P, Epp A, et al. Obstetrical anal sphincter injuries (OASIS): prevention, recognition, and repair. J Obstet Gynaecol Can. 2015;37(12):1131-48.
Return to footnote 178 referrer
Royal College of Obstetricians and Gynaecologists. Third- and fourth-degree perineal tears, management (green-top guideline no.29) [Internet]. London (UK): RCOG; 2015 [cited 2020 July 24]. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg29/
Return to footnote 179 referrer
Chalmers B, Omer-Hashi K. Female genital mutilation and obstetric care. Victoria (BC): Trafford Publishing; 2003.
Return to footnote 180 referrer
Sperstad JB, Tennfjord MK, Hilde G, Ellström-Engh M, Bø K. Diastasis recti abdominis during pregnancy and 12 months after childbirth: prevalence, risk factors and report of lumbopelvic pain. Br J Sports Med. 2016;50(17):1092-96.
Return to footnote 181 referrer
Fernandes da Mota PG, Pascoal AGBA, Carita AIAD, Bø K. Prevalence and risk factors of diastasis recti abdominis from late pregnancy to 6 months postpartum, and relationship with lumbo-pelvic pain. Man Ther. 2015; 2014;20(1):200-5.
Return to footnote 182 referrer
Rath A, Attali P, Dumas J, Goldlust D, Zhang J, Chevrel J. The abdominal linea alba: an anatomo-radiologic and biomechanical study. Surg Radiol Anat. 1996;18(4):281-8.
Return to footnote 183 referrer
Lee DG, Lee LJ, McLaughlin L. Stability, continence and breathing: The role of fascia following pregnancy and delivery. J Bodywork Move Ther. 2008;12(4):333-48.
Return to footnote 184 referrer
Gilleard WL, Brown JMM. Structure and function of the abdominal muscles in primigravid subjects during pregnancy and the immediate postbirth period. Phys Ther. 1996;76(7):750-62.
Return to footnote 185 referrer
Benjamin DR, van de Water ATM, Peiris CL. Effects of exercise on diastasis of the rectus abdominis muscle in the antenatal and postnatal periods: a systematic review. Physiotherapy. 2014;100(1):1-8.
Return to footnote 186 referrer
Hauck K, Davey R. Diastasis recti and pregnancy: 'closing the gap' between current treatment practices and clinical evidence [Internet]. Toronto (ON): Toronto Physiotherapy; 2015 [cited 2020 July 24]. Available from: http://torontophysiotherapy.ca/diastasis-recti-and-pregnancy-closing-the-gap-between-current-treatment-practices-and-clinical-evidence/
Return to footnote 187 referrer
Spitznagle TM, Leong FC, Van Dillen LR. Prevalence of diastasis recti abdominis in a urogynecological patient population. J Pelvic Floor Dysfunct. 2007;18(3):321-8.
Return to footnote 188 referrer
Kamel DM, Yousif AM. Neuromuscular electrical stimulation and strength recovery of postnatal diastasis recti abdominis muscles. Ann Rehabil Med. 2017;41(3):465-74.
Return to footnote 189 referrer
Thompson D, Berger H, Feig D, Gagnon R, Kader T, Keely E, et al. Diabetes and pregnancy. Can J Diabetes. 2013;37(1):S168-83.
Return to footnote 190 referrer
Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis. 2011;8(6):A124.
Return to footnote 191 referrer
Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314-19.
Return to footnote 192 referrer
Keely E. An opportunity not to be missed - how do we improve postpartum screening rates for women with gestational diabetes? Diabetes Metab Res. 2012;28(4):312-6.
Return to footnote 193 referrer
Berger H, Gagnon R, Sermer M, Basso M, Bos H, Brown RN, et al. Diabetes in Pregnancy. J Obstet Gynaecol Can. 2016;38(7):667-79.e1.
Return to footnote 194 referrer
Matias SL, Dewey KG, Quesenberry CP, Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr. 2014;99(1):115-21.
Return to footnote 195 referrer
Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92(3):574-84.
Return to footnote 196 referrer
Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, et al. Diabetes and pregnancy. Can J Diabetes. 2018;42(3):S255–82.
Return to footnote 197 referrer
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
Return to footnote 198 referrer
National Collaborating Centre for Women's and Children's Health. Antenatal care routine care for the healthy pregnant woman [Internet]. London (UK): RCOG Press; 2008 [cited 2020 July 24]. Available from: https://www.nice.org.uk/guidance/cg62/evidence/evidence-tables-from-the-2003-version-pdf-196748322
Return to footnote 199 referrer
Aslan E, Fynes M. Symphysial pelvic dysfunction. Curr Opin Gynecol Obstet. 2007;19(2):133-9.
Return to footnote 200 referrer
Vleeming A, Albert HB, Östgaard HC, Sturesson B, Stuge B, Sahlgrenska akademin, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794-819.
Return to footnote 201 referrer
Leadbetter RE, Mawer D, Lindow SW. Symphysis pubis dysfunction: a review of the literature. J Matern Fetal Neonatal Med. 2004;16(6):349-54.
Return to footnote 202 referrer
Kanakaris NK, Roberts CS, Giannoudis PV. Pregnancy-related pelvic girdle pain: an update. BMC Med. 2011;9(1):15.
Return to footnote 203 referrer
Wu WH, Meijer OG, Uegaki K, Mens JMA, van Dieën JH, Wuisman PIJM, et al. Pregnancy-related pelvic girdle pain (PPP), I: terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13(7):575-89.
Return to footnote 204 referrer
Jain S, Eedarapalli P, Jamjute P, Sawdy R. Symphysis pubis dysfunction: a practical approach to management. Obstet Gynaecol. 2006;8(3):153-8.
Return to footnote 205 referrer
Pelvic Obstetric and Gynaecological Physiotherapy. Pregnancy-related pelvic girdle pain (PGP) – for health professionals [Internet]. London (UK): Chartered Society of Physiotherapy; 2015 [cited 2020 July 24]. Available from: http://pogp.csp.org.uk/publications/pregnancy-related-pelvic-girdle-pain-pgp-health-professionals
Return to footnote 206 referrer
Khorashadi L, Petscavage JM, Richardson ML. Postpartum symphysis pubis diastasis. Radiol Case Rep. 2011;6(3):542.
Return to footnote 207 referrer
Health Service Executive, Therapy Professions Committee, Institute of Obstetricians and Gynaecologists. Clinical practice guideline: management of pelvic girdle pain in pregnancy and post-partum [Internet]. Dublin (IE): Health Service Executive; 2012 [cited 2020 July 24]. Available from: https://www.hse.ie/eng/services/publications/clinical-strategy-and-programmes/management-of-pelvic-girdle-pain-in-pregnancy-and-post-partum.pdf
Return to footnote 208 referrer
Britnell SJ, Cole JV, Isherwood L, Sran MM, Stan MM, Britnell N, et al. Postural health in women: the role of physiotherapy. J Obstet Gynaecol Can. 2005;27(5):493-510.
Return to footnote 209 referrer
Canadian Institute for Health Information. Quick stats: childbirth indicators by place of residence. [Internet]. Ottawa (ON): CIHI; 2020 [cited 2020 July 24]. Available from: https://apps.cihi.ca/mstrapp/asp/Main.aspx?Server=apmstrextprd_i&project=Quick%20Stats&uid=pce_pub_en&pwd=&evt=2048001&visualizationMode=0&documentID=029DB170438205AEBCC75B8673CCE822
Return to footnote 210 referrer
Nielsen PE, Deering SH, Galan HL. Operative vaginal delivery. In: Gabbe SG, Niebyl JR, Simpson JL, et al. Obstetrics: normal and problem pregnancies. 7th ed. Philadelphia (PA): Elsevier; 2017.
Return to footnote 211 referrer
Meyer S, Hohlfeld P, Achtari C, Russolo A, Grandi P. Birth trauma: short and long term effects of forceps delivery compared with spontaneous delivery on various pelvic floor parameters. BJOG. 2000;107(11):1360-5.
Return to footnote 212 referrer
Briggs J. The Joanna Briggs Institute best practice information sheet: The effectiveness of pelvic floor muscle exercises on urinary incontinence in women following childbirth. Nurs Health Sci. 2011;13(3):378-81.
Return to footnote 213 referrer
Brubaker L. Postpartum urinary incontinence: the problem is clear, but there is no simple solution. BMJ. 2002;324(7348):1227-8.
Return to footnote 214 referrer
Public Health Agency of Canada. Data tables - the maternity experiences survey (MES) 2006-2007 [Internet]. Ottawa (ON): PHAC; 2006 [cited 2020 July 24]. Available from: http://www.phac-aspc.gc.ca/rhs-ssg/pdf/tab-eng.pdf
Return to footnote 215 referrer
Boyle R, Hay-Smith EJC, Cody JD, Mørkved S. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2012;10:CD007471.
Return to footnote 216 referrer
Magali R, Ross S. Conservative management of urinary incontinence. J Obstet Gynaecol Can. 2018;40(2):E119-25.
Return to footnote 217 referrer
Yip S, Sahota D, Pang M, Chang A. Postpartum urinary retention. Acta Obstet Gynecol Scand. 2004;83(10):881-91.
Return to footnote 218 referrer
Carley ME, Carley JM, Vasdev G, Lesnick TG, Webb MJ, Ramin KD, et al. Factors that are associated with clinically overt postpartum urinary retention after vaginal delivery. Obstet Gynaecol. 2002;187(2):430-3.
Return to footnote 219 referrer
Health Service Executive, Institute of Obstetricians and Gynaecologists. Clinical practice guideline: management of urinary retention in pregnancy, post-partum and after gynaecological surgery [Internet]. Dublin (IE): Health Service Executive; 2018 [cited 2020 July 24]. Available from: https://rcpi-live-cdn.s3.amazonaws.com/wp-content/uploads/2018/06/UR-guidelines-for-clinical-care-pathway-17.05.18.pdf
Return to footnote 220 referrer
Lim JL. Post-partum voiding dysfunction and urinary retention: post-partum voiding dysfunction. Aust N Z J Obstet Gynaecol. 2010;50(6):502-5.
Return to footnote 221 referrer
Groutz A, Levin I, Gold R, Pauzner D, Lessing JB, Gordon D. Protracted postpartum urinary retention: the importance of early diagnosis and timely intervention. Neurourol Urodyn. 2011;30(1):83-6.
Return to footnote 222 referrer
Rizvi RM, Khan ZS, Khan ZS. Diagnosis and management of postpartum urinary retention. Int J Obstet Gynaecol. 2005;91(1):71-2.
Return to footnote 223 referrer
Borello-France D, Burgio KL, Richter HE, Zyczynski H, FitzGerald MP, Whitehead W, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynaecol. 2006;108(4):863-72.
Return to footnote 224 referrer
Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ. 2002;166(3):326-30.
Return to footnote 225 referrer
Watson J, Hermann S, Johnson B. Developing a policy to support breastfeeding in women who are hospitalized and acutely ill. Nurs Womens Health. 2013;17(3):188-96.
Return to footnote 226 referrer
Roger Hixon Ontario Milk Bank. Evidence for the use of donor milk [Internet]. Toronto (ON): Sinai Health; 2018 [cited 2020 July 24]. Available from: https://www.milkbankontario.ca/research-and-resources/evidence-for-the-use-of-donor-milk/
Return to footnote 227 referrer
Sachs HC, American Academy of Pediatrics, Committee On Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796-809.
Return to footnote 228 referrer
Money D, Allen VM. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can. 2016;38(12):S326-35.
Return to footnote 229 referrer
Centers for Disease Control and Prevention. Group B strep (GBS): clinical overview [Internet]. Atlanta (GA): CDC; 2020 [cited 2020 July 29]. Available from: https://www.cdc.gov/groupbstrep/clinicians/index.html
Return to footnote 230 referrer
Sgro M, Campbell DM, Sankaran K, Tran D, Yudin M. Early-onset neonatal sepsis and meningitis [Internet]. Ottawa (ON): CPSP; 2012 [cited 2020 July 29]. Available from: https://www.cpsp.cps.ca/surveillance/study-etude/early-onset-neonatal-sepsis-and-meningitis
Return to footnote 231 referrer
American Academy of Pediatrics, American Heart Association. Neonatal resuscitation textbook. 6 th ed. Itasca (IL): American Academy of Pediatrics; 2011.
Thomas SL, Heaton J, Makaryus AN. Physiology, cardiovascular murmurs. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 July 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525958/
Return to footnote 233 referrer
Mejia E, Dhuper S. Innocent murmur. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 July 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507849/
Return to footnote 234 referrer
Wight N, Marinelli KA, Academy of Breastfeeding Medicine. ABM clinical protocol #1: guidelines for blood glucose monitoring and treatment of hypoglycemia in term and late-preterm neonates, revised 2014. Breastfeed Med. 2014;9(4):173-9.
Return to footnote 235 referrer
Nicholl R. What is the normal range of blood glucose concentrations in healthy term newborns? Arch Dis Child. 2003;88(3):238-9.
Return to footnote 236 referrer
Adamkin DH, Papile L, Baley JE, Bhutani VK, Carlo WA, Kumar P, et al. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127(3):575-9.
Return to footnote 237 referrer
Narvey MR, Marks SD, Canadian Paediatric Society, Fetus and Newborn Committee. The screening and management of newborns at risk for low blood glucose. Paediatr Child Health. 2019;24(8):536-44.
Return to footnote 238 referrer
Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Obstet Gynaecol. 2008;198(2):194.e1-5.
Return to footnote 239 referrer
Jefferies AL, Canadian Paediatric Society, Fetus and Newborn Committee. Selective serotonin reuptake inhibitors in pregnancy and infant outcomes. Paediatr Child Health. 2011;16(9):562.
Return to footnote 240 referrer
Hudak ML, Tan RC, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-60.
Return to footnote 241 referrer
Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Vazquez DM. Selective serotonin reuptake iInhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol. 2005;25(9):595-604.
Return to footnote 242 referrer
Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293(19):2372-83.
Return to footnote 243 referrer
Perinatal Services BC. Newborn guideline 13: newborn nursing care pathway [Internet]. Vancouver (BC): PSBC; 2013 [cited 2020 July 29]. Available from: http://www.perinatalservicesbc.ca/Documents/Guidelines-Standards/Newborn/NewbornNursingCarePathway.pdf
Return to footnote 244 referrer
Public Health Agency of Canada. Perinatal health indicators for Canada 2017. Ottawa (ON): PHAC; 2017.
Return to footnote 245 referrer
Organization for Economic Cooperation and Development. Health at a glance 2013: OECD indicators. 7th ed. Washington (DC): Organization for Economic Cooperation & Development; 2013.
Return to footnote 246 referrer
Stavis RL. Small-for-gestational-age (SGA) infant [Internet]. Kenilworth (NJ): Merck Manual Professional Version; 2019 [cited 2020 July 29]. Available from: http://www.merckmanuals.com/en-ca/professional/pediatrics/perinatal-problems/small-for-gestational-age-sga-infant
Return to footnote 247 referrer
Zhang X, PhD, Decker A, MD, Platt RW, PhD, Kramer MS, MD. How big is too big? The perinatal consequences of fetal macrosomia. Obstet Gynaecol. 2008;198(5):517.e1-6.
Return to footnote 248 referrer
Mayo Clinic. Fetal macrosomia [Internet]. Scottsdale (AZ): Mayo Clinic; 2020 [cited 2020 July 29]. Available from: http://www.mayoclinic.org/diseases-conditions/fetal-macrosomia/basics/complications/con-20035423
Return to footnote 249 referrer
KC K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(2):14-20.
Return to footnote 250 referrer
Martins F, Oppolzer D, Santos C, Barroso M, Gallardo E. Opioid use in pregnant women and neonatal abstinence syndrome - a review of the literature. Toxics. 2019;7(1):9.
Return to footnote 251 referrer
British Columbia Centre on Substance Use, B.C. Ministry of Health, B.C. Ministry of Mental Health and Addictions, Perinatal Services BC. The treatment of opioid use disorder during pregnancy [Internet]. Vancouver (BC): BCCSU; 2018 [cited 2020 July 29.] Available from: http://www.bccsu.ca/wp-content/uploads/2018/06/OUD-Pregnancy.pdf
Return to footnote 252 referrer
Lacaze-Masmonteil T, O'Flaherty P, Canadian Paediatric Society, Fetus and Newborn Committee. Managing infants born to mothers who have used opioids during pregnancy [Internet]. Ottawa (ON): CPS; 2018 [cited 2020 July 29]. Available from: https://cps.ca/en/documents/position/opioids-during-pregnancy
Return to footnote 253 referrer
Wong S, Ordean A, Kahan M, Society of Obstetricians and Gynecologists of Canada. Substance use in pregnancy. Int J Gynaecol Obstet. 2011;114(2):190-202.
Return to footnote 254 referrer
Whyte RK, Hilliard RI, Jefferies AL, Peliowski-Davidovich A, Sorokan ST, Whyte HEA. Safe discharge of the late preterm infant. Paediatr Child Health. 2010;15(10):655-60.
Return to footnote 255 referrer
Boyle EM, Johnson S, Manktelow B, Seaton SE, Draper ES, Smith LK, et al. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: a prospective population-based study. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F479-85.
Return to footnote 256 referrer
Parikh LI, Reddy UM, Männistö T, Mendola P, Sjaarda L, Hinkle S, et al. Neonatal outcomes in early term birth. Obstet Gynaecol. 2014;211(3):265.e1-11.
Return to footnote 257 referrer
Ramphul M, Kennelly M, Burke G, Murphy D. Risk factors and morbidity associated with suboptimal instrument placement at instrumental delivery: observational study nested within the instrumental delivery & ultrasound randomised controlled trial. BJOG. 2015;122(4):558-63.
Return to footnote 258 referrer
Edozien LC. Towards safe practice in instrumental vaginal delivery. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):639-55.
Return to footnote 259 referrer
Demissie K, Rhoads GG, Smulian JC, Balasubramanian BA, Gandhi K, Joseph KS, et al. Operative vaginal delivery and neonatal and infant adverse outcomes: population based retrospective analysis. BMJ. 2004;329(7456):24-6.
Return to footnote 260 referrer
Lemacks J, Fowles K, Mateus A, Thomas K. Insights from parents about caring for a child with birth defects. Int J Environ Res Public Health. 2013;10(8):3465-82.
Return to footnote 261 referrer
Stone MB, Botto LD, Feldkamp ML, Smith KR, Roling L, Yamashiro D, et al. Improving quality of life of children with oral clefts: perspectives of parents. J Craniofac Surg. 2010;21(5):1358-64.
Return to footnote 262 referrer
Kaye, C.I. Reducing cultural barriers to the provision of genetic services in South Texas, final report [Internet]. San Antonio (TX): Maternal and Child Health Library – Georgetown University; 2003 [cited 2020 July 29]. Available from: https://www.mchlibrary.org/MCHBfinalreports/docs/fr00109.pdf
Return to footnote 263 referrer
Shilling V, Morris C, Thompson-Coon J, Ukoumunne O, Rogers M, Logan S. Peer support for parents of children with chronic disabling conditions: a systematic review of quantitative and qualitative studies. Dev Med Child Neurol. 2013;55(7):602-9.
Return to footnote 264 referrer
O'Brien K, Bracht M, Robson K, Ye XY, Mirea L, Cruz M, et al. Evaluation of the family integrated care model of neonatal intensive care: a cluster randomized controlled trial in Canada and Australia. BMC Pediatr. 2015;15(1):210.
Return to footnote 265 referrer
Family Integrated Care. Program development [Internet]. Tampa (FL): FICare; 2016 [cited 2020 July 29]. Available from: http://familyintegratedcare.com/implementing-ficare/program- development/
Return to footnote 266 referrer
Cooper LG, Gooding JS, Gallagher J, Sternesky L, Ledsky R, Berns SD. Impact of a family-centered care initiative on NICU care, staff and families. J Perinatol. 2007;27(S2):S32-7.
Return to footnote 267 referrer
Griffin T, Celenza J. Family-centered care for the newborn: The delivery room and beyond. New York (NY): Springer Publishing Company; 2014.
Return to footnote 268 referrer
O'Brien K, Robson K, Bracht M, Cruz M, Lui K, Alvaro R, et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health. 2018;2(4):245-54.
Return to footnote 269 referrer
Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103-28.
Return to footnote 270 referrer
Craig JW, Glick C, Phillips R, Hall SL, Smith J, Browne J. Recommendations for involving the family in developmental care of the NICU baby. J Perinatol. 2015;35(S1):S5-8.
Return to footnote 271 referrer
Family Integrated Care. About FICare [Internet]. Tampa (FL): FICare; 2017 [cited 2020 July 29]. Available from: http://familyintegratedcare.com/about-ficare/
Return to footnote 272 referrer
Banerjee J. Are single family rooms the future for neonatal units? Lancet Child Adolesc Health. 2019;3(3):130-1.
Return to footnote 273 referrer
Banerjee J, Aloysius A, Platonos K, Deierl A. Family centred care and family delivered care – What are we talking about? J Neonatal Nurs. 2018;24(1):8-12.
Return to footnote 274 referrer
Stevens D, Thompson P, Helseth C, Pottala J. Mounting evidence favoring single-family room neonatal intensive care. J Neonatal Perinatal Med. 2015;8(3):177-8.
Return to footnote 275 referrer
van Veenendaal NR, Heideman WH, Limpens J, van der Lee JH, van Goudoever JB, van Kempen AAMW, et al. Hospitalising preterm infants in single family rooms versus open bay units: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2019;3(3):147-57.
Return to footnote 276 referrer
Harris DD, Shepley MM, White RD, Kolberg KJS, Harrell JW. The impact of single family room design on patients and caregivers: executive summary. J Perinatol. 2006;26:38-48.
Return to footnote 277 referrer
Als H, McAnulty GB. The newborn individualized developmental care and assessment program (NIDCAP) with kangaroo mother care (KMC): comprehensive care for preterm infants. Curr Womens Health Rev. 2011;7(3):288.
Return to footnote 278 referrer
Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2006(2):CD001814.
Return to footnote 279 referrer
Trajkovski S, Schmied V, Vickers M, Jackson D. Neonatal nurses' perspectives of family‐centred care: a qualitative study. J Clin Nurs. 2012;21(17‐18):2477-87.
Return to footnote 280 referrer
Ramezani T, Hadian Shirazi Z, Sabet Sarvestani R, Moattari M. Family-centered care in neonatal intensive care unit: a concept analysis. Int J Community Based Nurs Midwifery. 2014;2(4):268-78.
Return to footnote 281 referrer
Kearvell H, Grant J. Getting connected: how nurses can support mother/infant attachment in the neonatal intensive care unit. Aust J Adv Nurs. 2010;27(3):75-82.
Return to footnote 282 referrer
National Institute for Health and Care Excellence. Neonatal specialist care [Internet]. London (UK): NICE; 2010 [cited 2020 Jul 29]. Available from: https://www.nice.org.uk/guidance/qs4
Return to footnote 283 referrer
Association of Women's Health, Obstetric and Neonatal Nursing. Guidelines for professional registered nurse staffing for perinatal units: executive summary. J Obstet Gynecol Neonatal Nurs. 2011;40(1):131-4.
Return to footnote 284 referrer
White RD. Development of care in the NICU. J Perinatol. 2014;34(3):174-5.
Return to footnote 285 referrer
Joshi A, Chyou P-, Tirmizi Z, Gross J. Web camera use in the neonatal intensive care unit: impact on nursing workflow. Clin Med Res. 2016;14(1):1-6.
Return to footnote 286 referrer
Best Start Resource Centre. Prenatal education: key messages for Ontario [Internet]. Toronto (ON): Best Start; 2016 [cited 2020 July 29]. Available from: https://resources.beststart.org/product/e49e-prenatal-education-key-messages-for-ontario-website/
Return to footnote 287 referrer
Irwin LG, Siddiqi A, Hertzman C. Early child development : a powerful equalizer: final report for the World Health Organization's Commission on the Social Determinants of Health [Internet]. Geneva (CH): WHO; 2007 [cited 2020 July 29]. Available from: https://apps.who.int/iris/handle/10665/69729
Return to footnote 288 referrer
BC Women's Hospital & Health Centre, British Columbia Centre of Excellence for Women's Health. A women's health strategy for British Columbia: advance the health of girls and women [Internet]. Vancouver (BC): BCCEWH; 2004 [cited 2020 July 29]. Available from: http://bccewh.bc.ca/wp-content/uploads/2012/05/2004_Advancing-Health-Girls-Women.pdf
Return to footnote 289 referrer
British Columbia Ministry of Health. Recommendations from the maternity care enhancement project [Internet]. Vancouver (BC): British Columbia Ministry of Health; 2004 [cited 2020 July 29]. Available from: https://www.health.gov.bc.ca/library/publications/year/2004/mcep_recommend_dec2004.pdf
Return to footnote 290 referrer
Public Health Agency of Canada. Population health approach: the organizing framework [Internet]. Ottawa (ON): PHAC; 2013 [cited 2020 July 29]. Available from: https://cbpp-pcpe.phac-aspc.gc.ca/population-health-approach-organizing-framework/
Return to footnote 291 referrer
Champlain Maternal Newborn Regional Program. Mapping of the maternal-newborn care spectrum in the Champlain and southeast LHINS: final report and recommendations [Internet]. Ottawa (ON): CMNRP; 2017 [cited 2020 July 31]. Available from: https://instantweb.secure-form2.com/uploads/documents//Mapping_of_Maternal_Newborn_Service_Final_Report_Nov20_2017.pdf
Return to footnote 292 referrer
Best Start Resource Centre. Abuse in pregnancy: information and strategies for the prenatal educator [Internet]. Toronto (ON): Best Start; 2015 [cited 2020 July 31]. Available from: https://resources.beststart.org/wp-content/uploads/2016/01/H04-E.pdf
Return to footnote 293 referrer
Beydoun HA, Al-Sahab B, Beydoun MA, Tamim H. Intimate partner violence as a risk factor for postpartum depression among Canadian women in the maternity experience survey. Ann Epidemiol. 2010;20(8):575-83.
Return to footnote 294 referrer
World Health Organization. Child maltreatment: the health sector responds [Internet]. Geneva (CH): WHO; 2017 [cited 2020 July 31]. Available from: https://www.who.int/violence_injury_prevention/violence/child/Child_maltreatment_infographic_EN.pdf?ua=1
Return to footnote 295 referrer
Sinha M. Family violence in Canada: a statistical profile, 2010 [Internet]. Ottawa (ON): Statistics Canada; 2012 [cited 2020 July 31]. Available from: https://www150.statcan.gc.ca/n1/en/pub/85-002-x/2012001/article/11643-eng.pdf?st=swG0oiwz
Return to footnote 296 referrer
Paolozza C, Packard B, Kulkarni C. A call to action on behalf of maltreated infants and toddlers in Canada [Internet]. Toronto (ON) Infant Mental Health Promotion; 2017 [cited 2020 July 31]. Available from: http://www.imhpromotion.ca/Resources/CalltoAction
Return to footnote 297 referrer
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14(4):245-58.
Return to footnote 298 referrer
World Health Organization. Responding to intimate partner violence and sexual violence against women: WHO clinical and policy guidelines [Internet]. Geneva (CH): WHO; 2013 [cited 2020 July 31]. Available from: https://www.who.int/reproductivehealth/publications/violence/9789241548595/en/
Return to footnote 299 referrer
VEGA Family Violence Project. VEGA family violence education resources [Internet]. Hamilton (ON): McMaster University; 2019 [cited 2020 July 31]. Available from: https://vegaproject.mcmaster.ca/
Return to footnote 300 referrer
World Health Organization. Health care for women subjected to intimate partner violence or sexual violence: a clinical handbook [Internet]. Geneva (CH): WHO; 2014 [cited 2020 July 31]. Available from: https://www.who.int/reproductivehealth/publications/violence/vaw-clinical-handbook/en/
Return to footnote 301 referrer
Wilson RD. Pre-conception folic acid and multivitamin supplementation for the primary and secondary prevention of neural tube defects and other folic acid-sensitive congenital anomalies. J Obstet Gynaecol Can. 2015;37(6):534-49.
Return to footnote 302 referrer
Tarasuk V, Mitchell A, Dachner N. Household food insecurity in Canada, 2014 [Internet]. Toronto (ON): University of Toronto; 2016 [cited 2020 July 31]. Available from: https://proof.utoronto.ca/wp-content/uploads/2016/04/Household-Food-Insecurity-in-Canada-2014.pdf
Return to footnote 303 referrer
Dietitians of Canada. Addressing household food insecurity in Canada - position statement and recommendations. Can J Diet Pract Res. 2016;77(3):159.
Return to footnote 304 referrer
O'Connor DL, Blake J, Bell R, Bowen A, Callum J, Fenton S, et al. Canadian consensus on female nutrition: adolescence, reproduction, menopause, and beyond. J Obstet Gynaecol Can. 2016;38(6):508-554.e18.
Return to footnote 305 referrer
Incollingo Rodriguez AC, Dunkel Schetter C, Tomiyama AJ. Weight stigma among pregnant and postpartum women: A new context of stigmatization. Stigma Health. 5(2):209-16.
Return to footnote 306 referrer
Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11(1):99-137.
Return to footnote 307 referrer
Nusbaum MRH, Gamble G, Skinner B, Heiman J. The high prevalence of sexual concerns among women seeking routine gynecological care. J Fam Pract. 2000;49(3):229-32.
Return to footnote 308 referrer
Basson R, Leiblum S, Brotto L, Derogatis L, Fourcroy J, Fugl-Meyer K, et al. Definitions of women's sexual dysfunction reconsidered: advocating expansion and revision. J Psychosom Obstet Gynaecol. 2003;24(4):221-9.
Return to footnote 309 referrer
Von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: results from the sexual preferences questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
Return to footnote 310 referrer
von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
Return to footnote 311 referrer
Hicks TL, Goodall SF, Quattrone EM, Lydon-Rochelle MT. Postpartum sexual functioning and method of delivery: summary of the evidence. J Midwifery Womens Health. 2004;49(5):430-6.
Return to footnote 312 referrer
Buhling KJ, Schmidt S, Robinson JN, Klapp C, Siebert G, Dudenhausen JW. Rate of dyspareunia after delivery in primiparae according to mode of delivery. Eur J Obstet Gynecol Reprod Biol. 2006;124(1):42-6.
Return to footnote 313 referrer
Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-7.
Return to footnote 314 referrer
Barrett G, Peacock J, Victor CR, Isaac. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-11.
Return to footnote 315 referrer
Black A, Francoeur D, Rowe T. Canadian contraception consensus. J Obstet Gynaecol. Can 2004 April 2004;26(4):347-87.
Return to footnote 316 referrer
Society of Obstetricians and Gynaecologists of Canada. Postpartum – contraception [Internet]. Ottawa (ON): SOGC; 2020 [cited 2020 July 31]. Available from: https://www.pregnancyinfo.ca/postpartum/postpartum/contraception/
Return to footnote 317 referrer
World Alliance for Breastfeeding Action. LAM – The lactational amenorrhea method [Internet]. Penang (MY): WABA; 2017 [cited 2020 July 31]. Available from: http://www.waba.org.my/resources/lam/
Return to footnote 318 referrer
Guttmann A, Shulman R, Manuel D. Improving accountability for children's health: immunization registries and public reporting of coverage in Canada. Paediatr Child Health. 2011;16(1):16-8.
Return to footnote 319 referrer
Public Health Agency of Canada. Message from the Chief Public Health Officer of Canada: national immunization awareness week - April 23 to 30, 2016 [Internet]. Ottawa (ON): PHAC; 2016 [cited 2020 July 31]. Available from: https://www.canada.ca/en/public-health/news/2016/04/message-from-the-chief-public-health-officer-of-canada-national-immunization-awareness-week-april-23-to-30-2016.html
Return to footnote 320 referrer
Public Health Agency of Canada. Vaccine coverage in Canadian children: highlights from the 2013 childhood national immunization coverage survey (cNICS) [Internet]. Ottawa (ON): PHAC; 2016 [cited 2020 July 31]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-coverage-canadian-children-highlights-2013-childhood-national-immunization-coverage-survey.html?_ga=1.10659505.462930771.1474382704
Return to footnote 321 referrer
Public Health Agency of Canada. Guidelines for measles outbreak in Canada [Internet]. Ottawa (ON): PHAC; 2013 [cited 2020 July 31]. Available from: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2013-39/guidelines-prevention-control-measles-outbreaks-canada.html
Return to footnote 322 referrer
Wilson SE, Deeks SL, Seo CY, Lim GH, Fediurek J, Crowcroft NS. Trends in medical and nonmedical immunization exemptions to measles-containing vaccine in Ontario: an annual cross-sectional assessment of students from school years 2002/03 to 2012/13. CMAJ 2015;3(3):E317-23.
Return to footnote 323 referrer
Busby C, Jacobs A, Muthukumaran R. In need of a booster: how to improve childhood vaccination coverage in Canada [Internet]. Toronto (ON): C.D. Howe Institute; 2017 [cited 2020 July 31]. Available from: https://www.cdhowe.org/sites/default/files/attachments/research_papers/mixed/Commentary_477.pdf
Return to footnote 324 referrer
MacDonald NE, Desai S, Gerstein B. Working with vaccine-hesitant parents: an update. Paediatr Child Health. 2018;23(8):561.
Return to footnote 325 referrer
De Vries R, Wiegers TA, Smulders B, Teijlingen Ev. The Dutch obstetrical system: vanguard of the future in maternity care. In: Davis-Floyd R, Barclay L, Daviss, BA, Tritten J. Birth models that work. Berkeley (CA): University of California Press; 2019.
Return to footnote 326 referrer
Expatica. Having a baby in France [Internet]. Haarlem (NL): Expatica; 2020 [cited 2020 July 31]. Available from: https://www.expatica.com/fr/healthcare/womens-health/having-a-baby-in-france-107664/
Return to footnote 327 referrer
Reproductive Care Program of Nova Scotia. Healthy babies, healthy families: postpartum & postnatal guidelines [Internet]. Halifax (NS): Nova Scotia Department of Health; 2012 [cited 2020 July 31]. Available from: https://novascotia.ca/dhw/publications/Public-Health-Education/Postpartum%20Guidelines.pdf
Return to footnote 328 referrer
Monarch. About us [Internet]. Ottawa (ON): Monarch Centres – Maternal and Newborn Health; 2015 [cited 2020 July 31]. Available from: http://www.monarchcentre.ca/us/
Return to footnote 329 referrer
Children's Health Policy Centre. Nurse-family partnership is being tested in Canada [Internet]. Vancouver (BC): Simon Fraser University; 2020 [cited 2020 July 31]. Available from: https://childhealthpolicy.ca/nurse-family-partnership/
Return to footnote 330 referrer
Nurse-Family Partnership Canada. Nurse-family partnership Canada [Internet]. Hamilton (ON): McMaster University; 2015 [cited 2020 July 31]. Available from: https://nfp.mcmaster.ca
Return to footnote 331 referrer
Public Health Agency of Canada. Canada prenatal nutrition program (CPNP) [Internet]. Ottawa (ON): PHAC; 2020 [cited 2020 July 31]. Available from: https://www.canada.ca/en/public-health/services/health-promotion/childhood-adolescence/programs-initiatives/canada-prenatal-nutrition-program-cpnp.html
Return to footnote 332 referrer
Public Health Agency of Canada. Community action program for children (CAPC) [Internet]. Ottawa (ON): PHAC; 2020 [cited 2020 July 31]. Available from: https://www.canada.ca/en/public-health/services/health-promotion/childhood-adolescence/programs-initiatives/community-action-program-children-capc.html
Return to footnote 333 referrer
Association of American Medical Colleges. Implementing curricular and institutional climate changes to improve health care for individuals who are LGBT, gender nonconforming, or born with DSD [Internet]. Washington (DC): AAMA; 2014 [cited 2020 June 24]. Available from: https://store.aamc.org/implementing-curricular-and-institutional-climate-changes-to-improve-health-care-for-individuals-who-are-lgbt-gender-nonconforming-or-born-with-dsd-a-resource-for-medical-educators.html
Return to footnote 334 referrer
Page details
You are using an outdated browser. Please upgrade your browser or activate Google Chrome Frame to improve your experience.
- Foundations

Service Directory
Service points.
- Prepare for a surgery
- Health centres and clinics
- Oncology Centre
- Provincial Programs
- Research and Training
Direct Access
- Blood tests
- Cardiac Rehabilitation
- Clinics by appointment
- Mental Health and Addiction Services
- MotivAction Youth Clinic
- New Brunswick Fetal Alcohol Spectrum Disorder (FASD) Centre of Excellence
- Palliative Care
- Patient Connect NB
- Public Health
- Respiratory Health Clinics
- Sexual Health
- Smoking Cessation Clinics
- Spiritual care
- The Single Entry Point
- Youth Services
Interactive Network Map

- Prepare for a Surgery
- Browse the Service Directory
- Patient Guide
- Visitor Guide
- Guidelines for Visitors and Designated Support Persons
- Wearing a mask in our service points
- Patient Experience
- Patient Rights and Responsibilities
- Privacy Statement
- Palliative care
- Enduring Power of Attorney for Personal Care and Health Care Directives (Living Will)
- Medical Assistance in Dying
- Information requests
- Questions or comments
- Paying invoices online
Visiting Hours
For most of our units, there is no restriction on visiting hours.
However, visitors must respect the hours of rest, from 9 p.m. to 6 a.m.
Find out more
Chronic Illness
- Take Control of Diabetes
- Respiratory Health
- A healthier diet
- Guide and recipes for introducing solid foods
- Omega 3 Fatty Acids and Pregnancy
- COVID-19: Advice
- Preventing infections
- Sexually transmitted and blood-borne infections
- Smoke-Free Together
- The Importance of Your Medication List
- About Our Name and Logo
- Official languages
- Quality, Patient Safety and Performance Improvement
- Improvements to Vitalité Health Network's infrastructures
- Ethics at Vitalité Health Network
- Publications
- Our partners
- Diversity, equity, inclusion, and accessibility
Administration
- Our Purpose and Values
- Board of Directors
- Leadership Team
- The voice of our leaders
- Organizational Chart
- Administrative by-laws
Prenatal and postnatal home visits
Mothers can be visited by a nurse and a dietitian at home before and after birth.
The goal of visits is to:
- Help future mothers to have a healthy pregnancy;
- Support parents and families;
- Strengthen parental role and attachment with their baby;
- Contribute to the growth and development of children from 0 to 2 years;
- Direct parents to appropriate community resources;
- Provide coupons for milk and vitamins.
Prenatal home visits
Who can receive this service.
- Adolescent, 19 or under and pregnant for the first time
- Education level;
- Use of tobacco;
- Interest in prenatal classes;
- Prior prenatal experience.
How to request a home visit
- Referral from a professional or a family member or friend.

Postnatal home visits
- Adolescent, 19 and under who is a first-time parent
- A handicap or health problem;
- Factors having a negative impact on the child’s development;
- Social or environmental factors putting the child at risk.
- Be referred by the Public Health nurse who assesses each new mother at the hospital to determine whether she can receive postnatal services from Public Health or other organizations.
- Call Public Health .
Quick Links

Why do nurse home visits stop a few weeks after giving birth? Extending them to 2 years benefits the whole family
The Erdi Foundation Child Health Equity (COVID-19) Scholar, Centre for Community Child Health | Honorary, Department of Paediatrics, University of Melbourne | Team Leader / Senior Research Officer, Murdoch Children's Research Institute
Director of the Translational Research and Social Innovation group, School of Nursing and Midwifery, Western Sydney University
Director, Center for Community Child Health Royal Children's Hospital; Professor, Department of Paediatrics, University of Melbourne; Theme Director Population Health, Murdoch Children's Research Institute
Disclosure statement
right@home is supported by the state governments of Victoria and Tasmania, the Ian Potter Foundation, Sabemo Trust, Sidney Myer fund, the Vincent Fairfax Family Foundation, and the National Health and Medical Research Council (NHMRC, 1079148). The MCRI administered the research grant for the study and provided infrastructural support to its staff but played no role in the conduct or analysis of the trial. Research at the MCRI is supported by the Victorian Government's Operational Infrastructure Support Program. SG was supported by NHMRC Practitioner Fellowship (1155290). The “right@home” sustained nurse home visiting trial is a research collaboration between the Australian Research Alliance for Children and Youth (ARACY); the Translational Research and Social Innovation (TReSI) Group at Western Sydney University; and the Centre for Community Child Health (CCCH), which is a department of The Royal Children's Hospital and a research group of Murdoch Children’s Research Institute. Ownership of the right@home implementation and support license, which is purchased by Australian state governments for roll out for fidelity support, is shared between institutes.
The MECSH home visiting program upon which right@home is based is trademarked and licenced by Western Sydney University and was developed by UNSW Australia. Western Sydney University is a member of the right@home consortium that receives funding from Australian state governments to support the implementation of the program. Western Sydney University also licenses the MECSH program to government and non-government providers of home visiting services in the UK and USA.
Sharon Goldfeld receives funding from ARC and NHMRC.
Western Sydney University provides funding as a member of The Conversation AU.
View all partners
Bringing home a new baby can be one of the most exciting and stressful times in your life. A nurse might visit a couple of times, then other than routine check-ups at the nurse’s office, you’re largely on your own.
Some people have a particularly hard time with a new baby because the challenges of parenting come on top of existing adversity, such as financial hardship, or poor physical or mental health.
Experiencing adversity from when a baby is conceived can affect the child’s health and development as they grow older. So rather than stopping nurse visits a week or two after bringing a new baby home, we investigated whether extending these visits from pregnancy until children turned two made a difference.
The nurse visits focused on areas fundamental for children’s development: how a parent cares for and responds to their child, and their home environment.
We found home visits with nurses helps parenting and family relationships, and women’s mental health, wellbeing and confidence.
What happens when the nurse visits?
Sustained nurse home visiting provides intensive services in a family’s home during pregnancy and the first two years of the child’s life. During this time, the child’s brain develops more rapidly than any other time in their life.
Read more: How do I know if my child is developing normally?
International studies of sustained nurse home visiting programs show they can help families with parenting, children’s behaviour and academic skills. However, most have only measured impacts up to when children turn three.
Programs differ depending on how they work to support families. They generally engage highly-trained nurses who can listen without judgement, offer practical, evidence-informed advice, and remind parents they’re doing a good job.
Our randomised controlled trial of right@home is Australia’s longest and largest trial of nurse home-visiting, starting in 2013.
The program supports parents with evidence-based techniques that promote parenting that responds to the child’s needs, safe homes, regular routines, and children’s learning and language development. The program starts in pregnancy and offers 25 home visits (60-90 minutes each) with a specially trained nurse until the children turn two.
The right@home program was designed for delivery through Australia’s existing child and family health nursing services, which are free for families with children from birth to school age. These existing services typically offer a handful of appointments (of around 20-40 minutes) that mostly take place in local clinics. Some also offer more intensive services.

We invited women into the right@home study if they were experiencing two or more factors that can make it more difficult to parent. These include having low social support, poor physical or emotional health, or no household employment. We found almost 40% of pregnant women experienced at least two of these factors.
In total, 722 women and families living across Victoria and Tasmania took part in the study. Half were randomly allocated (like tossing a coin) to receive the right@home program and half received their local child and family health nursing service.
Read more: Parents have the biggest influence over their child's language and emotional development
What did we find?
Researchers who were separate to the nurse teams interviewed the families twice a year (one at home and one by phone) until children started school.
When the right@home program ended (at children’s second birthdays), the evaluation showed it offered benefits over and above the usual services. Parents had more confidence and skills in caring for their children, responding sensitively and providing a nurturing and stimulating home.
This pattern continued . At three years, parents who received the right@home program reported benefits to their mental health, wellbeing, and self-confidence.

Our latest paper, published in PLOS ONE , shows that right@home offered lasting impacts to four and five years, which is two and three years after the program ended.
Some 5-10% more families had regular mealtimes, bedtimes and bedtime routines by the time the children turned five.
Around 9% more women reported very good health and parenting confidence. The proportions of women experiencing stress, and emotional abuse from an intimate partner were 7% and 11% lower, respectively.
There were additional benefits for children’s and women’s mental health, parenting, and women’s wellbeing, quality of life and relationship with their child. These impacts were evident for families regardless of where they lived across the seven regional and metropolitan areas in the two states.
Read more: Having problems with your kid's tantrums, bed-wetting or withdrawal? Here's when to get help
Levelling the playing field for kids
A goal of the program is to address the challenging circumstances that disrupt parenting and affect children’s health and development.
If Australia did this, and provided support according to need, we could reduce children’s poor developmental outcomes by 50%-70% .
Providing equitable support is especially important as we emerge from the COVID pandemic , which has disproportionately affected families already experiencing adversity.

Almost no other public health program delivered during the early years has evidence of such a broad range of gains.
Our economic evaluation of right@home at three years showed delivering these benefits through the right@home program costs A$7,700 extra per family compared with the usual service.
Research from the US shows the benefits of similar programs accrue for families and taxpayers over a child’s lifetime, producing positive returns on investment, from improved mental health and more employment, among other benefits.
Australia is fortunate to have nationwide child and family health nursing services. These are the perfect platform for delivering an extended program like right@home. When so few programs make a difference for families experiencing adversity, we should maximise the reach of those that do.
Diana Harris, Lead for Knowledge Translation at the Australian Research Alliance for Children & Youth, coauthored this article.
Read more: Stressed about managing your child's behaviour? Here are four things every parent should know
- Child development
- Infant health
- Maternal health
- Maternal and child health
- disadvantage
- Maternal mental health

Biocloud Project Manager - Australian Biocommons

Director, Defence and Security

Opportunities with the new CIEHF

School of Social Sciences – Public Policy and International Relations opportunities

Deputy Editor - Technology
Jump to navigation
- Bahasa Malaysia

Home visits in the early period after the birth of a baby
What is the issue?
Health problems for mothers and babies commonly occur or become apparent in the weeks following the birth. For the mothers these include postpartum haemorrhage (substitute excessive blood loss), fever and infection, abdominal and back pain, abnormal discharge (heavy or smelly vaginal discharge), thromboembolism (a blood clot), and urinary tract complications (being unable to control the urge to pee), as well as psychological and mental health problems such as postnatal depression. Mothers may also need support to establish breastfeeding. Babies are at risk of death related to infections (babies may be badly affected by infections), asphyxia (difficulties in breathing, caused by lack of oxygen), and preterm birth (being born prematurely).
Why is this important?
Home visits by health professionals or lay supporters in the early postpartum period may prevent health problems from becoming long-term, with effects on women, their babies, and their families. This review looked at different home-visiting schedules in the weeks following the birth.
What evidence did we find?
We included 16 randomised trials with data for 12,080 women. Some trials focused on physical checks of the mother and newborn, while others provided support for breastfeeding, and one included the provision of practical support with housework and childcare. They were carried out in both high-resource countries and low-resource settings where women receiving usual care may not have received additional postnatal care after early hospital discharge.
The trials focused on three broad types of comparisons: schedules involving more versus fewer postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus facility postnatal check-ups (eight studies). In all but four of the included studies, postnatal care at home was delivered by healthcare professionals. For most of our outcomes, only one or two studies provided data. Overall, our results were inconsistent.
The evidence was very uncertain about whether home visits reduced newborn deaths or serious health problems with the mother. Women's physical and psychological health were not improved with more intensive schedules of home visits although more individualised care improved women's mental health in one study and maternal satisfaction was slightly better in two studies. Overall, babies may be less likely to have additional medical care if their mothers received more postnatal home visits. More home visits may have encouraged more women to exclusively breastfeed their babies and women to be more satisfied with their postnatal care. The different outcomes reported in different studies, how the outcomes were measured, and the considerable variation in the interventions and control conditions across studies were limitations of this review. The certainty of the evidence was generally found to be low or very low according to the GRADE criteria.
What does this mean?
Increasing the number of postnatal home visits may promote infant health and exclusive breastfeeding and more individualised care may improve outcomes for women. More research is needed before any particular schedule of postnatal care can be recommended.
The evidence is very uncertain about the effect of home visits on maternal and neonatal mortality. Individualised care as part of a package of home visits probably improves depression scores at four months and increasing the frequency of home visits may improve exclusive breastfeeding rates and infant healthcare utilisation. Maternal satisfaction may also be better with home visits compared to hospital check-ups. Overall, the certainty of evidence was found to be low and findings were not consistent among studies and comparisons. Further well designed RCTs evaluating this complex intervention will be required to formulate the optimal package.
Maternal complications, including psychological/mental health problems and neonatal morbidity, have commonly been observed in the postpartum period. Home visits by health professionals or lay supporters in the weeks following birth may prevent health problems from becoming chronic, with long-term effects. This is an update of a review last published in 2017.
The primary objective of this review is to assess the effects of different home-visiting schedules on maternal and newborn mortality during the early postpartum period. The review focuses on the frequency of home visits (how many home visits in total), the timing (when visits started, e.g. within 48 hours of the birth), duration (when visits ended), intensity (how many visits per week), and different types of home-visiting interventions.
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (19 May 2021), and checked reference lists of retrieved studies.
Randomised controlled trials (RCTs) (including cluster- , quasi-RCTs and studies available only as abstracts) comparing different home-visiting interventions that enrolled participants in the early postpartum period (up to 42 days after birth) were eligible for inclusion. We excluded studies in which women were enrolled and received an intervention during the antenatal period (even if the intervention continued into the postnatal period), and studies recruiting only women from specific high-risk groups (e.g. women with alcohol or drug problems).
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We used the GRADE approach to assess the certainty of the evidence.
We included 16 randomised trials with data for 12,080 women. The trials were carried out in countries across the world, in both high- and low-resource settings. In low-resource settings, women receiving usual care may have received no additional postnatal care after early hospital discharge.
The interventions and controls varied considerably across studies. Trials focused on three broad types of comparisons, as detailed below. In all but four of the included studies, postnatal care at home was delivered by healthcare professionals. The aim of all interventions was broadly to assess the well-being of mothers and babies, and to provide education and support. However, some interventions had more specific aims, such as to encourage breastfeeding, or to provide practical support.
For most of our outcomes, only one or two studies provided data, and results were inconsistent overall. All studies had several domains with high or unclear risk of bias.
More versus fewer home visits (five studies, 2102 women)
The evidence is very uncertain about whether home visits have any effect on maternal and neonatal mortality (very low-certainty evidence). Mean postnatal depression scores as measured with the Edinburgh Postnatal Depression Scale (EPDS) may be slightly higher (worse) with more home visits, though the difference in scores was not clinically meaningful (mean difference (MD) 1.02, 95% confidence interval (CI) 0.25 to 1.79; two studies, 767 women; low-certainty evidence). Two separate analyses indicated conflicting results for maternal satisfaction (both low-certainty evidence); one indicated that there may be benefit with fewer visits, though the 95% CI just crossed the line of no effect (risk ratio (RR) 0.96, 95% CI 0.90 to 1.02; two studies, 862 women). However, in another study, the additional support provided by health visitors was associated with increased mean satisfaction scores (MD 14.70, 95% CI 8.43 to 20.97; one study, 280 women; low-certainty evidence). Infant healthcare utilisation may be decreased with more home visits (RR 0.48, 95% CI 0.36 to 0.64; four studies, 1365 infants) and exclusive breastfeeding at six weeks may be increased (RR 1.17, 95% CI 1.01 to 1.36; three studies, 960 women; low-certainty evidence). Serious neonatal morbidity up to six months was not reported in any trial.
Different models of postnatal care (three studies, 4394 women)
In a cluster-RCT comparing usual care with individualised care by midwives, extended up to three months after the birth, there may be little or no difference in neonatal mortality (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 infants). The proportion of women with EPDS scores ≥ 13 at four months is probably reduced with individualised care (RR 0.68, 95% CI 0.53 to 0.86; one study, 1295 women). One study suggests there may be little to no difference between home visits and telephone screening in neonatal morbidity up to 28 days (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 women). In a different study, there was no difference between breastfeeding promotion and routine visits in exclusive breastfeeding rates at six months (RR 1.47, 95% CI 0.81 to 2.69; one study, 656 women).
Home versus facility-based postnatal care (eight studies, 5179 women)
The evidence suggests there may be little to no difference in postnatal depression rates at 42 days postpartum and also as measured on an EPDS scale at 60 days. Maternal satisfaction with postnatal care may be better with home visits (RR 1.36, 95% CI 1.14 to 1.62; three studies, 2368 women). There may be little to no difference in infant emergency health care visits or infant hospital readmissions (RR 1.15, 95% CI 0.95 to 1.38; three studies, 3257 women) or in exclusive breastfeeding at two weeks (RR 1.05, 95% CI 0.93 to 1.18; 1 study, 513 women).

Model Profiles
Family connects.
Family Connects is designed to support whole-person, integrated health for all families of newborns at a moment of life-changing transition. Family Connects nurses are trained to carefully assess newborns and mothers and to discuss concrete next steps to address opportunities and concerns, including seeking immediate medical care when necessary. They work together with families and build from identified strengths to connect them to community resources that meet their needs and preferences. Family Connects nurses also keep the whole family in mind, recommending appropriate mental health services or medical care for other family members as needed—and they follow up to make sure families’ needs are met. Family Connects aims to reach at least 60 to 70 percent of families with newborns in each community it serves.
What is the model’s approach to providing home visiting services?
All families in an affiliated community are offered up to three home visits during the first 3 weeks after birth. Home visits last up to 2 hours each. A registered nurse connects with a family in their home shortly after birth to assess the child’s and birthing person’s physical health status (as applicable), assess unique family strengths and challenges, and respond to immediate family needs.
Who is implementing the model?
Home Visitors
Family Connects was implemented by 129 full-time equivalent (FTE) home visitors in 2022. Home visiting providers are Registered Nurses with up-to-date professional licenses, who function within the state’s Nurse Practice Act and are trained to provide newborn, caregiver, and family health and psychosocial assessments. Full-time home visitors typically enroll six to eight new families per week.
Supervisors
Family Connects was implemented by 29 FTE nurse supervisors in 2022.
Where is the model implemented?
Family Connects operated in 35 local agencies across 16 states in 2022.

Families Served Through Evidence-Based Home Visiting in 2022
<1% American Indian Alaska Native
<1% Native Hawaiian Pacific Islander
3% Multiple
13% Another race
35% Hispanic or Latino
65% Not Hispanic or Latino
0% 1-2 years
0% 3-5 years
Child insurance status
36% Private
Caregiver primary language
76% English
20% Spanish
4% Another language
Caregiver education
15% No HS diploma
29% HS diploma or GED
22% Some college or training
33% Bachelor's degree or higher
Caregiver age
22% 18-24 years
28% 25-30 years
49% >30 years
Stay up to date on the latest home visiting information.

What to Expect… At Your Home Visit and One Week Visit
by thebirthcenter | Sep 15, 2018 | Pregnancy, Birth, Postpartum, and Motherhood | 0 comments

by Justine Deputy, RN, MSN
Photo: Heather (TBC Mama), photo by Nicole Dimotsis of Femina Photo & Design
Although The Birth Center has early discharge after birth, you can read more about that here , you receive a plethora of follow-up that exceeds the standard follow up care for postpartum mothers. Two main pieces of this follow up care are the home visit and one week visit. Here is what you can expect at each:
The Home Visit
First things first, when the nurse arrives at your house for you home visit, she is not looking for a sparkling clean house. She is looking for a Mama resting. Because that is what a Mama with a two to three-day-old baby should be doing! Do not worry about what your house looks like or what you look like. You should be resting and taking care of yourself and baby, the other things can wait.
Home visits are completed between 36 and 72 hours after birth. The nurse will do an assessment on both mom and baby. For mom, this includes things such as vital signs, how breastfeeding is going, how mom is healing, is mom resting enough, and how mom is feeling emotionally during those early days. Some of the things the nurse will do for baby are vital signs, check the umbilical cord, talk with you about the pees and the poops , do the p ulse oximetry screening and the HMD blood screening, and check for jaundice.
This visit usually takes about one to two hours. The only thing you need to do is be there! If you have questions you want to remember for the visit, it can be helpful to jot them down so you don’t forget them when the nurse is there. The lack of sleep and adjustment to having a baby home can make it hard to remember anything off the top of your head.
The One Week Visit
At one week, we will see you back at our office to check how things are going and how you and baby are doing. The nurse will assess mom and baby, discuss and/or observe breastfeeding, and talk with you about any questions or concerns you might have. Again, make sure to write down those questions!
The nurse will take a look at some things like the baby’s weight, jaundice, and umbilical cord. She will also complete the second HMD screening and baby’s hearing screening. For mom, she will check things such as vital signs, the uterus, healing of any stitches, and how you are doing with the adjustment of having a new baby at home. The nurse will share tips and information related to breastfeeding, family planning, and peer support. There is plenty of time to ask any questions you might have.
We love getting to see you and your new baby during these visits!
After the one week, we will see you for a six-week visit, but we may see you sooner for a lactation visit or when you stop by for mom’s group. Stayed tuned for what to expect at those visits in a future blog!
Submit a Comment Cancel reply
You must be logged in to post a comment.
Critics concerned over lack of minimum care hours in Alberta continuing care regulations
Health minister says standard of daily care hadn't been updated since 1985.

Social Sharing
Alberta's new continuing care regulations come into effect April 1, but critics are questioning why the new rules do not prescribe how many hours of care residents of a facility should receive.
The Nursing Homes Operations Regulation said each resident of a facility should get a minimum 1.9 hours of nursing and personal care each day, but the new Continuing Care Act Regulations are silent on the issue.
"Until now, if an Albertan wanted to be assured that their loved one would get a minimum standard of care, it was in the law," NDP Leader Rachel Notley said at a news conference Tuesday. "Now there is no guarantee."
The new regulations were approved by cabinet in a closed-door meeting. They were published online at the end of February.
- Alberta health minister says 'proper procedures' were followed for patient taken to hotel instead of care home
- Edmonton hospital patient had been hoping for a care home. He wound up at a hotel instead
Health Minister Adriana LaGrange said the government is providing funding so continuing care providers can provide an average of 3.62 hours of care a day.
Notley said that's not the same as putting those minimum standards into law. She said the hours would be discretionary and subject to one-on-one discussions between the government and contracted caregivers.
The United Nurses of Alberta are also warning about the change. UNA president Heather Smith said in a news release that a report commissioned by the province three years ago recommended a jump in the hours of care each resident received.
Smith said the new regulations go in the other direction.
"We are moving to zero hours of care," she said. "This is extremely dangerous."
Flexibility for operators
During Tuesday's question period, LaGrange said the standard of 1.9 hours of daily care hadn't been updated since 1985. She said the government consulted with operators, workers and residents.
"We heard that changes were needed to provide better flexibility and to allow the operators to develop staffing plans that really meet the needs of not just their facilities but also their patients unique needs," LaGrange told the legislature.
Notley said Ontario has prescribed four hours of care per resident each day. She wants the government to change the regulations to include that amount as a minimum.
Questions about the new continuing care standards come as the government continues to face criticism over the case of Blair Canniff, an Edmonton stroke patient who was taken to a motel in Leduc by a contracted service provider after he was discharged from the Royal Alexandra Hospital earlier this month.
Canniff is paralyzed on one side and uses a wheelchair. He says he was told he was going to a long-term care facility. Instead, he was dropped at the motel, where he was given fast food meals and struggled to get into the bathroom and the bed. He was moved back to the hospital a week later.
LaGrange said during the review of her budget estimates Tuesday that Canniff chose to go to the Travelodge in Leduc after discussions with Alberta Health Services.
While she was questioned by reporters later, LaGrange said she had received information about Canniff's case from Alberta Health Services. She said the non-profit provider Contentment Social Services chose to move him to the motel.
ABOUT THE AUTHOR

Provincial affairs reporter
Michelle Bellefontaine covers the Alberta legislature for CBC News in Edmonton. She has also worked as a reporter in the Maritimes and in northern Canada.
Related Stories
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cochrane Database Syst Rev
Schedules for home visits in the early postpartum period
Maternal complications including psychological and mental health problems and neonatal morbidity have been commonly observed in the postpartum period. Home visits by health professionals or lay supporters in the weeks following the birth may prevent health problems from becoming chronic with long‐term effects on women, their babies, and their families.
To assess outcomes for women and babies of different home‐visiting schedules during the early postpartum period. The review focuses on the frequency of home visits, the duration (when visits ended) and intensity, and on different types of home‐visiting interventions.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (28 January 2013) and reference lists of retrieved articles.

Selection criteria
Randomised controlled trials (RCTs) (including cluster‐RCTs) comparing different types of home‐visiting interventions enrolling participants in the early postpartum period (up to 42 days after birth). We excluded studies in which women were enrolled and received an intervention during the antenatal period (even if the intervention continued into the postnatal period) and studies recruiting only women from specific high‐risk groups. (e.g. women with alcohol or drug problems).
Data collection and analysis
Study eligibility was assessed by at least two review authors. Data extraction and assessment of risk of bias were carried out independently by at least two review authors. Data were entered into Review Manager software.
Main results
We included data from 12 randomised trials with data for more than 11,000 women. The trials were carried out in countries across the world, and in both high‐ and low‐resource settings. In low‐resource settings women receiving usual care may have received no additional postnatal care after early hospital discharge.
The interventions and control conditions varied considerably across studies with trials focusing on three broad types of comparisons: schedules involving more versus fewer postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus hospital clinic postnatal check‐ups (four studies). In all but two of the included studies, postnatal care at home was delivered by healthcare professionals. The aim of all interventions was broadly to assess the wellbeing of mothers and babies, and to provide education and support, although some interventions had more specific aims such as to encourage breastfeeding, or to provide practical support.
For most of our outcomes only one or two studies provided data, and overall results were inconsistent.
There was no evidence that home visits were associated with improvements in maternal and neonatal mortality, and no consistent evidence that more postnatal visits at home were associated with improvements in maternal health. More intensive schedules of home visits did not appear to improve maternal psychological health and results from two studies suggested that women receiving more visits had higher mean depression scores. The reason for this finding was not clear. In a cluster randomised trial comparing usual care with individualised care by midwives extended up to three months after the birth, the proportions of women with Edinburgh postnatal depression scale (EPDS) scores ≥ 13 at four months was reduced in the individualised care group (RR 0.68, 95% CI 0.53 to 0.86). There was some evidence that postnatal care at home may reduce infant health service utilisation in the weeks following the birth, and that more home visits may encourage more women to exclusively breastfeed their babies. There was some evidence that home visits are associated with increased maternal satisfaction with postnatal care.
Authors' conclusions
Increasing the number of postnatal home visits may promote infant health and maternal satisfaction and more individualised care may improve outcomes for women, although overall findings in different studies were not consistent. The frequency, timing, duration and intensity of such postnatal care visits should be based upon local and individual needs. Further well designed RCTs evaluating this complex intervention will be required to formulate the optimal package.
Plain language summary
Home visits in the early period after the birth of a baby
Health problems for mothers and babies commonly occur or become apparent in the weeks following the birth. For the mothers these include postpartum haemorrhage, fever and infection, abdominal and back pain, abnormal discharge, thromboembolism, and urinary tract complications, as well as psychological and mental health problems such as postnatal depression. Mothers may also need support to establish breastfeeding. Babies are at risk of death related to infections, asphyxia, and preterm birth. Home visits by health professionals or lay supporters in the early postpartum period may prevent health problems from becoming long‐term, with effects on women, their babies, and their families. This review looked at different home‐visiting schedules in the weeks following the birth.
We included 12 randomised trials with data for more than 11,000 women. Some trials focused on physical checks of the mother and newborn, while others provided support for breastfeeding, and one included the provision of practical support with housework and childcare. They were carried out in both high‐resource countries and low‐resource settings where women receiving usual care may not have received additional postnatal care after early hospital discharge.
The trials focused on three broad types of comparisons: schedules involving more versus less postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus hospital clinic postnatal check‐ups (four studies). In all but two of the included studies postnatal care at home was delivered by healthcare professionals. For most of our outcomes only one or two studies provided data and overall results were inconsistent.
There was no evidence that home visits were associated with reduced newborn deaths or serious health problems for the mothers. Women's physical and psychological health were not improved with more intensive schedules of home visits although more individualised care improved women's mental health in one study. Overall, babies were less likely to have emergency medical care if their mothers received more postnatal home visits. More home visits may have encouraged more women to exclusively breastfeed their babies. The different outcomes reported in different studies, how the outcomes were measured, and the considerable variation in the interventions and control conditions across studies were limitations of this review. The studies were of mixed quality as regards risk of bias.
More research is needed before any particular schedule of postnatal care can be recommended
Description of the condition
The postpartum period, defined by the World Health Organization (WHO) as the period from childbirth to the 42nd day following delivery ( WHO 2005 ), is critical for both mothers and newborns. An estimated 529,000 maternal deaths occur worldwide each year because of pregnancy‐related complications in the antenatal, intrapartum, and postpartum periods, especially in resource‐limited settings ( WHO 2005 ).These deaths are often sudden and unpredictable, with 11% to 17% occurring during childbirth itself and 50% to 71% occurring during the postpartum period ( WHO 2005 ). Maternal health problems commonly observed in the postpartum period include postpartum haemorrhage, fever, abdominal and back pain, abnormal discharge, puerperal genital infection, thromboembolic disease, and urinary tract complications ( Bashour 2008 ), as well as psychological and mental health problems such as postnatal depression. The postpartum period is also critical for newborns. Every year approximately 3.7 million babies die in the first four weeks of life. Most of these infants are born in developing countries and most die at home. Nearly 40% of all deaths of children younger than five years old occur within the first 28 days of life (neonatal or newborn period). Just three causes—infections, asphyxia, and preterm birth—account for nearly 80% of these deaths ( WHO/UNICEF 2009 ). Moreover, the postpartum period is a time of transition for women and their families, who are adjusting on physical, psychological, and social levels ( Shaw 2006 ). In most developed countries, postpartum hospital stays are often shorter than 48 hours following a vaginal birth; thus most postpartum care is provided in community and ambulatory‐care settings. Early intervention in the postpartum period may prevent health problems from becoming chronic with long‐term effects on women, their babies, and their families.
Description of the intervention
The purpose of a home‐visiting program is to provide support at home for mothers, babies, and families by health professionals or skilled attendants. However, a single clearly defined methodology for this intervention does not exist. Further, the term "home visiting" is used differently in various contexts ( AAP 2009 ). Since the 1970s, the length of hospital stay after childbirth has fallen dramatically in many high‐resource settings. Early postnatal discharge of healthy mothers and term infants does not appear to have adverse effects on breastfeeding or maternal depression when women are offered at least one nurse‐midwife home visit after discharge ( Brown 2002 ). Home‐visiting programs provide breastfeeding and hygiene education, parenting and child health instruction, and general support to families, successfully addressing many of the barriers to access including transportation issues, initiation of timely care, and completeness of services ( AAP 1998 ; AAP 2009 ). Several trials have assessed the impact of home‐visiting programs, especially effects on child abuse and neglect in vulnerable families ( Donovan 2007 ; Olds 1997 ; Quinlivan 2003 ). Others focused on the effectiveness and cost‐effectiveness of intensive home‐visiting programs ( Barlow 2006 ; Carabin 2005 ; McIntosh 2009 ). Some home‐visiting programs have specifically targeted high risk groups such as women suffering domestic abuse (intimate partner violence) or families that are economically or socially disadvantaged. Home‐visiting programs for high risk groups or those by child health nurses may include components during pregnancy and may continue over many months or years; such programs are outside the scope of this review and have been addressed in other Cochrane reviews ( Bennett 2008 ; Jahanfar 2013 ; Macdonald 2008 ; Turnbull 2012 ). In this review we focus on the early postnatal period following discharge from hospital.
In 2009, WHO and the United Nations Children's Fund recommended home visits by a skilled attendant in resource‐limited settings. In high‐mortality settings and where access to facility‐based care is limited, at least two home visits are recommended for all home births: the first visit should occur within 24 hours of the birth, the second visit on day three, and if possible, a third visit should be made before the end of the first week of life (day seven). For babies born in a healthcare facility, the first home visit was recommended to be made as soon as possible after the mother and baby return home with remaining visits following the same schedule as for home births ( WHO/UNICEF 2009 ).
A recent review demonstrated the effectiveness of community‐based intervention packages in improving neonatal outcomes and reducing maternal and neonatal morbidity and mortality in resource‐limited settings; home visiting is the one of the main components in each of these intervention packages. This review offers encouraging evidence of the value of integrating maternal and newborn care in community settings ( Lassi 2010 ). We, therefore, did not include intervention packages of continuous care with components of antenatal or hospital care in our review.
How the intervention might work
In high‐resource settings healthy women and babies are frequently discharged from hospital within one or two days of the birth, and in low‐resource settings women may be discharged within hours of the birth or give birth at home ( Brown 2002 ). Potentially, home visits in the first few days of the birth by healthcare professions or trained support workers offer opportunities for assessment of the mother and newborn, health education, infant feeding support, emotional or practical support and, if necessary, referral to other health professionals or agencies ( Carabin 2005 ; Donovan 2007 ; Lassi 2010 ; Shaw 2006 ). Postpartum visits may prevent health problems developing or reduce their impact by early intervention or referral. Home visits have improved coverage of key maternal and newborn care practices such as early initiation of breastfeeding, exclusive breastfeeding, skin‐to‐skin contact, delayed bathing, attention to hygiene (e.g. hand washing and water quality), umbilical cord care, infant skin care. In addition, home visits may identify conditions that require additional care or check‐up, as well as counselling regarding when to take the mother and newborn to a healthcare facility ( WHO/UNICEF 2009 ). Home visits may involve not only the assessment of the mother and newborn for physical problems but also assessment of maternal mental health, family circumstances and the home environment.
Depending on the context, home visits may take a non‐judgmental and supportive role or a more directive approach in which the goals are to monitor family compliance with standards of parenting care and ensure the newborn's health and welfare.The type of approach used can influence the ability of the carers to engage mothers and newborns, resulting in acceptance or rejection of the help offered and potential for further disengagement ( Doggett 2005 ).
Why it is important to do this review
Despite many studies and reviews, evidence regarding the effectiveness of different types of home‐visiting programs in the early postnatal period is not sufficient. In some contexts once women have been discharged from hospital there may be no, or very limited postnatal follow‐up. In higher‐resource settings once women are at home, services may be provided by a range of health and social care agencies (newborn health visitors, social workers, paediatricians and general practitioners) and may be fragmented; postnatal home visits potentially allow continuity of care after hospital discharge and for the assessment and referral of the mother and newborn.
This review addresses the following questions: do different schedules of postpartum home‐visiting programs reduce maternal/neonatal mortality and morbidities, and if they do, what is the optimal schedule for postpartum home visits? This review includes reports evaluating the frequency, timing, duration and intensity of home visits.The optimal schedule has been set out by WHO/UNICEF 2009 , however, there was no clear evidence underpinning recommendations.
The primary objective of this review is to assess outcomes (maternal and newborn mortality) of different home‐visiting schedules during the early postpartum period. The review focuses on the frequency of home visits (how many home visits altogether), the timing (when visits started, e.g. within 48 hours of the birth), duration (when visits ended), intensity (how many visits per week), and different types of home‐visiting interventions.
Criteria for considering studies for this review
Types of studies.
We included studies that compared outcomes after home visits with outcomes of no home visits or different types of home‐visiting interventions; studies that used random or quasi‐random allocations of participants; and those in which the unit of allocation was the individual or group (cluster‐randomised). We also planned to include studies available only as abstracts, noting that these studies were awaiting assessment, pending publication of the full report. There was, however, no such study identified.
Types of participants
Eligible studies were ones that enrolled participants in the early postpartum period (up to 42 days after birth). We excluded studies in which women were enrolled and received an intervention during the antenatal period, even those in which the intervention continued into the postnatal period.
We planned to exclude studies that only recruited women from specific high‐risk groups (e.g. women identified with alcohol or drug problems) as interventions to support such women have been addressed elsewhere ( Turnbull 2012 ).
Types of interventions
Interventions included scheduled home visiting in the postpartum period (excluding studies with antenatal home visiting in which the visits continued over many months). Interventions were home visits with various frequency, timings, duration and intensity.
We planned to include studies with co‐intervention(s). Home visits may include outreach visits to non‐healthcare facilities. Trials including a group that did not receive home visits would have been eligible but would have been analysed separately.
Types of outcome measures
Primary outcomes.
- Maternal mortality at 42 days post birth.
- Neonatal mortality.
Secondary outcomes
Maternal outcomes.
- Maternal morbidities (postpartum haemorrhage, puerperal fever, abdominal and back pain, abnormal discharge, puerperal genital infection, thromboembolic disease, and urinary tract complications) within 42 days after birth.
- Maternal mental health (depression, anxiety) and related problems (intimate partner violence, drug use) at 42 days after birth.
- Satisfaction with overall care and service at 42 days after birth.
Neonatal outcomes
- Neonatal morbidities (pneumonia, upper respiratory tract infection, diarrhoea, septic meningitis, encephalopathy or cerebral injury, and jaundice) within 28 days after birth.
- Established feeding regimen (e.g. exclusive breastfeeding) at 28 days after birth.
- Incomplete immunisation.
- Failure to thrive, abuse, neglect, domestic violence from parents for any reason within 28 days after birth.
Search methods for identification of studies
Electronic searches.
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
- monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
- weekly searches of MEDLINE;
- weekly searches of Embase;
- handsearches of 30 journals and the proceedings of major conferences;
- weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
(1) references from published studies.
We searched the reference lists of relevant trials and reviews identified.
(2) Unpublished literature
We planned to contact the authors for more details about the published trials/ongoing trials.
We did not apply any language restrictions.
Selection of studies
Two review authors (NY and SN) independently assessed eligibility for inclusion for all studies identified as a result of the search strategy. We resolved discrepancies by discussion and by consulting a third review author (RM).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (NY and SN) extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into the Review Manager (RevMan) software ( RevMan 2012 ) and checked for accuracy.
If information regarding any of the above had been unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (TD and NY) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). We resolved any disagreement by discussion or by involving an additional assessor (RM).
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
- low risk of bias (any truly random process, e.g. random number table; computer random number generator);
- high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
- unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
- low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
- high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
- unclear risk of bias.
(3) Blinding (checking for possible performance and detection bias)
Blinding study participants and personnel from knowledge of which intervention a participant received is generally not feasible for this type of intervention. It may however, be possible to blind outcome assessment. We considered that studies were at low risk of bias for detection bias if outcome assessors were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
- low, high or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported we planned to re‐include missing data in the analyses. We were not, however, able to re‐include data, as the data were not available. We assessed methods as:
- low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
- high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
- unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
- low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
- high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
(6) Other sources of bias
We described for each included study any concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
- low risk of other bias;
- high risk of other bias;
- unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis .
Measures of treatment effect
Dichotomous data.
For dichotomous and categorical data, we used risk ratios (RR) with 95% confidence intervals (CI) and used the summary RR to combine trials that measured the same outcome. We had also planned to use the risk difference (RD).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI if outcomes were measured in the same way between trials. If required, we would have used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials.
We included cluster‐randomised trials in the analyses along with individually‐randomised trials. When including cluster trials, we adjusted their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ) using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial, from a similar trial or from a study of a similar population. Where we used ICCs from other sources, we reported this and planned to conduct sensitivity analyses to investigate the effect of variation in the ICC. We identified both cluster‐randomised trials and individually‐randomised trials, and we synthesised the relevant information provided there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Trials with multiple treatment arms
One trial with three arms has been included in this review as two separate studies ( Bashour 2008a ; Bashour 2008b ); to avoid double counting, the control group data (events and sample) were shared between the two study comparisons.
Dealing with missing data
For included studies, we noted levels of attrition as:
- low risk of bias (indicates no or low level of missing data on intention‐to‐treat basis);
- high risk of bias (indicates high level of missing data);
We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using Sensitivity analysis .
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We used the T², I² and Chi² statistics to examine heterogeneity among the trials in each analysis.
We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we had identified substantial heterogeneity, we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. We planned to assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it. In this version of the review, too few trials contributed data to allow us to carry out this planned analyses.
Data synthesis
We carried out statistical analysis using the RevMan software ( RevMan 2012 ). We used a fixed‐effect model for combining data where trials examined the same intervention, and the trials' populations and interventions were judged sufficiently similar. When there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or when substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. When the average treatment effect was not clinically meaningful, we did not combine trials. If we had identified heterogeneity for different types of study designs, we planned to carry out separate meta‐analysis by type of studies (individually‐randomised trial, cluster‐randomised trial).
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis according to the following clinically logical predefined groups.
- Initiation of the intervention (within 48 hours after birth or later).
- Duration of the intervention (< three weeks or ≥ three weeks).
- Intensity or frequency of the intervention (< one visit/week versus ≥ one visit/week.).
- Person doing the visit: medical professional versus skilled attendant.
- Parity: primiparity versus multiparity.
However, interventions in included trials were too heterogeneous to conduct subgroup analyses planned as above, and we therefore decided to conduct subgroup analyses by intensity/frequency of the intervention only in the comparison of more versus fewer home visits as below.
- Any number of home visits versus no home visit.
- Four or more home visits versus fewer than four visits.
- More home visits versus fewer home visits (both groups had more than four visits).
We planned subgroup analyses on both the primary and secondary outcomes.
For post hoc analyses, we considered a 99% CI excluding a zero treatment effect as statistically significant.
Also, when we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and where it was, used random‐effects analysis to produce it.
We assessed differences between subgroups using interaction tests available in RevMan 2012 .
Sensitivity analysis
We planned to perform sensitivity analyses to explore the effect of trial quality on the primary analysis and primary outcomes.
We planned to explore any risk of bias associated with a particular aspect of study quality (e.g. high risk of bias for allocation concealment) by sensitivity analyses.
Where we included cluster‐randomised trials, we planned to carry out sensitivity analysis using a range of values for intra class correlation coefficients.
Description of studies
Results of the search.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register identified 21 reports, and our additional searches of reference lists identified eight reports. Some studies resulted in multiple publications and one duplicate report. The 28 unique reports equated to 24 separate studies. After assessing eligibility we included 12 studies (one trial with three arms has been reported in this review as two separate studies ( Bashour 2008a ; Bashour 2008b )). We excluded 10 studies. Two reports are awaiting further assessment pending translation from the original Spanish ( Furnieles‐Paterna 2011 ; Salazar 2011 ) and more information about these trials is set out in Characteristics of studies awaiting classification tables.
Included studies
After assessing eligibility we included 12 randomised trials with a total of 11,287 women.
Three of the trials ( Christie 2011 ; Kronborg 2007 ; MacArthur 2002 ) were cluster‐randomised and health centres or healthcare staff were the units of randomisation. For these trials event rates and, or sample sizes have been adjusted in the analysis to take account of cluster design effect.
One of the trials included three arms; women in the intervention groups received either four or one home visits, while the control group received no visits. In order for us to set out the results for all three groups we have reported this trial as though it was two studies ( Bashour 2008a ; Bashour 2008b ). In the Data and analyses , women receiving four visits versus no visits are entered under Bashour 2008a ; whereas one versus no visits are compared in Bashour 2008b . The events and sample number for the control group have been divided between these comparisons to avoid double counting.
Results of trials were published between 1998 and 2012 although study data may have been collected some years before publication (e.g. in Ransjo‐Arvidson 1998 women were recruited between 1989 and 1992).
The studies were carried out in countries across the globe in both high‐ and low‐resource settings. Three studies were carried out the USA ( Escobar 2001 ; Lieu 2000 ; Paul 2012 ), three in the UK ( Christie 2011 ; MacArthur 2002 ; Morrell 2000 ), two in Canada ( Gagnon 2002 ; Steel 2003 ), and one each in Turkey ( Aksu 2010 ), Syria ( Bashour 2008a ; Bashour 2008b ), Denmark ( Kronborg 2007 ) and Zambia ( Ransjo‐Arvidson 1998 ). It is important to take the time and setting into account when interpreting results as routine practice varied across time and in different settings; for example, in the UK usual care may have involved up to seven home visits, whereas in other settings there may have been no postnatal care after hospital discharge.
The number and type of visits examined varied considerably across these trials, and control conditions also varied. Broadly, trials examined three types of comparisons: schedules involving more versus less postnatal home visits; schedules involving different models of care; and home versus hospital clinic postnatal follow‐up. In view of the complexity of interventions we have set out the main components of interventions and a description of control conditions in Table 4 .
1. Schedules involving more versus fewer home visits
In five of our included studies the main comparison was between women receiving more versus fewer home visits in the postnatal period.
Aksu 2010 examined the effect of one postnatal visit by a trained supporter versus no postnatal visits; Bashour 2008a ; Bashour 2008b compared four or one postnatal home visits from midwives versus no home visits following hospital discharge. Ransjo‐Arvidson 1998 compared four versus one midwife home visits. In these three studies, carried out in low‐resource settings, women may have received no additional postnatal care.
In contrast, Christie 2011 and Morrell 2000 examined the impact of additional care in settings where women already received more than four postnatal visits from midwives as part of usual care. Christie 2011 compared groups receiving six versus one health visitor visits (in addition to midwifery care) and Morrell 2000 examined the impact of up to 10 visits from lay supporters; again visits were provided in addition to routine midwifery care which was available to women in both intervention and control groups. (In the data and analysis tables we have separated studies where women in both groups received more than four home visits as the impact of interventions is likely to be different from that in settings where women received no, or very limited postnatal care.)
2. Schedules comparing different models of postnatal care
Three studies examined different ways of providing postnatal care.
Steel 2003 compared the effects of two visits by public health nurses in the early postnatal period compared with a telephone screening interview with discretionary nurse home visits.
In a cluster‐randomised trial Kronborg 2007 looked at the effects of more structured postnatal visits; women in the intervention group were visited between one and three times by health visitors who had attended special training on promoting and supporting breastfeeding. Women in the control group received usual care by health visitors who had not attended the breastfeeding courses.
MacArthur 2002 compared postnatal care that was adapted to the individual needs of women and home visits extended beyond the usual period of care (flexible visits up to 10 to 12 weeks postpartum). This was compared with usual care which involved a more rigid schedule of midwife home visits confined to the early postnatal period.
3. Home versus hospital postnatal care
Four of the included studies compared outcomes in women attending hospital clinics for postnatal checks and follow‐up (usual care) versus home visits by nurses ( Escobar 2001 ; Gagnon 2002 ; Lieu 2000 ; Paul 2012 ).
For all types of comparisons the purpose of visits was broadly similar: to assess the physical health and wellbeing of mothers and babies (with referral for further care where necessary), to promote and support breastfeeding, to assess maternal emotional wellbeing and to offer health education and support. In some cases the intervention focused on a particular aspect of care (e.g. breastfeeding), whereas other interventions were more general.
The outcomes measured in different studies varied. Most studies included some measure of maternal and infant health (although the particular outcomes measured, the way they were measured, and the time of follow‐up varied considerably between studies). Health service utilisation was also reported in a number of trials. Maternal emotional wellbeing and rates of breastfeeding were reported in some of the studies, and a minority reported maternal satisfaction with postnatal care. In the data and analyses tables we have set up analyses for all prespecified outcomes even where no studies have reported results. We did this in order to illustrate gaps in the evidence, and so that empty tables can be populated in updates of the review as more data become available.
Excluded studies
Ten studies identified by the searches were excluded after assessing the full trial reports. Two trials were excluded as they focused on outcomes in women following early hospital discharge after the birth rather than on different schedules of home visits for women discharged at the same time ( Boulvain 2004 ; Carty 1990 ). Two studies did not specifically examine postnatal home visits ( Gunn 1998 ; Simons 2001 ). One study, which recruited high‐risk women, involved intervention by child health nurses, rather than more general care of the mother and baby in the early postnatal period ( Izzo 2005 ). Quinlivan 2003 also focused on a high‐risk group rather than on the impact of different schedules of care. Three excluded studies examined complex interventions that included components delivered during the antenatal period ( Korfmacher 1999 ; Lumley 2006 ; Olds 2002 ). Finally, Stanwick 1982 was excluded for methodological reasons; there were major protocol deviations in this study, with many women in the intervention group failing to receive the intervention as planned, and analysis was carried out according to treatment received rather than by randomisation group (data were not available to allow us to restore women to their original randomisation group).
Risk of bias in included studies
The included studies were of mixed methodological quality; we were unable to carry out planned sensitivity analysis (temporarily excluding studies at high or unclear risk of bias for allocation concealment) as too few studies contributed data to allow any meaningful additional analysis.
Ten of the 12 included studies used methods to generate the randomisation sequence that we judged were at low risk of bias: seven used computer‐generated sequences or external trial randomisation services ( Aksu 2010 ; Escobar 2001 ; Gagnon 2002 ; Kronborg 2007 ; Lieu 2000 ; MacArthur 2002 ; Paul 2012 ) and three used random number tables ( Christie 2011 ; Morrell 2000 ; Steel 2003 ). In the Bashour 2008a ; Bashour 2008b trial, it was not clear how the randomisation sequence was decided, and the method used by Ransjo‐Arvidson 1998 was assessed as being at high risk of bias.
Concealment of group allocation at the point of randomisation was assessed as being at low risk of bias in nine of the studies; five trials reported using sequentially numbered, sealed envelopes to conceal allocation ( Bashour 2008a ; Bashour 2008b ; Escobar 2001 ; Lieu 2000 ; Morrell 2000 ; Steel 2003 ) and four used external randomisation services ( Christie 2011 ; Gagnon 2002 ; Kronborg 2007 ; MacArthur 2002 ). In the trials by Aksu 2010 , Paul 2012 , and Ransjo‐Arvidson 1998 the methods used to conceal allocation were not described, or were not clear.
Blinding women and care providers to this type of intervention is not generally feasible and no attempts to achieve blinding for these groups were described. All studies were judged to be at high risk of bias for this domain. It is possible that lack of blinding may have been an important source of bias.
In seven of the trials it was reported that outcome assessors were blind to group allocation ( Bashour 2008a ; Bashour 2008b ; Escobar 2001 ; Gagnon 2002 ; Lieu 2000 ; MacArthur 2002 ; Paul 2012 ; Steel 2003 ). However, where outcome data were assessed by interview, women may have revealed their treatment group and it was not clear whether or not blinding was successful; none of the trialists reported checking the success of blinding. Blinding of outcome assessors was either not attempted or not mentioned in the remaining five trials ( Aksu 2010 ; Christie 2011 ; Kronborg 2007 ; Morrell 2000 ; Ransjo‐Arvidson 1998 ).
Incomplete outcome data
In six of the included trials sample attrition and missing data did not appear to be important sources of bias (assessed as low or unclear risk of bias) ( Bashour 2008a ; Bashour 2008b ; Christie 2011 ; Escobar 2001 ; Lieu 2000 ; Paul 2012 ; Steel 2003 ). In some trials, although attrition was balanced across groups there was more than 10% loss to follow‐up; in the Aksu 2010 trial the response rate at four months postpartum was 82%; 16% were lost to follow‐up in the Kronborg 2007 study and 15% in the trials by Gagnon 2002 and Ransjo‐Arvidson 1998 . By four months postpartum more than 20% of the sample were lost to follow‐up in the MacArthur 2002 trial. Loss to follow‐up was not balanced in the intervention and control groups in the Morrell 2000 study; while the response rate was 83% for those women receiving additional postnatal visits it was only 75% in the control group.
Selective reporting
Assessing selective reporting bias is not easy without access to study protocols, and for all studies included in the review, risk of bias was assessed from published study reports. In most, but not all of the studies the primary outcomes were specified in the methods section and trialists reported results for these outcomes. We were unable to carry out planned investigation of possible publication bias by generating funnel plots as too few studies contributed data.
Other potential sources of bias
In most of the studies there were no other obvious sources of bias. In four of the trials there was some imbalance between groups at baseline ( Escobar 2001 ; Gagnon 2002 ; Lieu 2000 ; Morrell 2000 ). In the cluster trial reported by Christie 2011 , health visitors were the unit of randomisation and it appeared that there were differences between health visitors in terms of the number of women recruited to the trial and in their practices; the impact of these differences in individual practices is unclear. In the Steel 2003 study women were recruited in two study areas and usual practice was different in each area and this led to protocol deviations; again, it is not clear how this would affect results. Finally, in the Ransjo‐Arvidson 1998 trial much of the analysis related to the intervention group only, in addition, the nature of the intervention may have affected findings. Midwives asked women about their health as part of the intervention so women in the intervention group were asked repeatedly to identify health problems, whereas women in the control group were only asked as part of follow‐up assessments; this may have affected recall and introduced a risk of response bias.This trial may also have had the potential for publication bias because the publication date was more than six years after study completion.
We have set out the 'Risk of bias' assessments for individual studies in Figure 1 and for overall bias across all studies for different bias domains in Figure 2 .

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison 1: schedules involving more versus fewer home visits (five trials with 2102 women).
In five included studies the main comparison was between women receiving more versus less home visits in the postnatal period ( Aksu 2010 ; Bashour 2008a ; Bashour 2008b ; Christie 2011 ; Morrell 2000 ; Ransjo‐Arvidson 1998 ). One trial included three arms and to allow us to report findings for two different intervention groups this trial has been treated as two separate studies in this review ( Bashour 2008a ; Bashour 2008b ). One of the trials ( Christie 2011 ) was a cluster‐randomised trial and in the data and analyses tables we have used the effective sample size and event rates (adjusted for cluster design effect).
Aksu 2010 examined the effect of one postnatal visit versus no postnatal visits; Bashour 2008a ; Bashour 2008b four or one home visits versus none; Ransjo‐Arvidson 1998 four versus one home visits. Christie 2011 and Morrell 2000 examined the impact of additional care in settings where women already received more than four visits as part of usual care. Christie 2011 compared groups receiving six versus one health visitor visits (in addition to midwifery care) and Morrell 2000 up to 10 lay supporter visits versus no additional visits with routine midwifery care available to women in both intervention and control groups. (In the data and analysis tables we have separated studies where women in both groups received more than four home visits.)
For many of our prespecified outcomes only one or two studies contributed data and results were not always available for all women randomised. For each result we have specified the number of studies and women for whom data were available (for cluster‐randomised trials these are the adjusted figures). We anticipated that the treatment effect might differ in trials comparing different numbers of visits, we therefore used a random‐effects model for all analyses in this comparison.
Maternal mortality up to 42 days postpartum
Only one trial reported this outcome ( Christie 2011 ). There was no evidence of differences in maternal mortality between groups receiving additional health visitor visits compared with controls, with only one death in 951 women (risk ratio (RR) 2.46, 95% confidence interval (CI) 0.10 to 60.14, one study with 951 women) ( Analysis 1.1 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 1 Maternal mortality within 42 days post birth.
Neonatal mortality
Two trials reported on neonatal death ( Bashour 2008a ; Bashour 2008b ; Ransjo‐Arvidson 1998 ); there was no strong evidence that more visits were associated with fewer deaths. Pooled results showed no evidence of differences between intervention and control groups (average RR 0.99, 95% CI 0.26 to 3.69, two studies, 1281 women), similarly women receiving four or one visits versus none, or four visits versus one had similar numbers of neonatal deaths (RR 3.06, 95% CI 0.37 to 25.39, one study, 873 women; and, RR 0.48, 95% CI 0.09 to 2.60, one study, 408 women, respectively) ( Analysis 1.2 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 2 Neonatal mortality.
Severe maternal morbidity
Two studies reported this outcome, Bashour 2008a ; Bashour 2008b reported the number of women seeking medical help for a health problem and Ransjo‐Arvidson 1998 the number of women where a doctor had identified a problem up to 42 days. The numbers of women with problems were very similar in intervention and control groups and there was no evidence of differences between groups either overall, or for women receiving different patterns of visits (overall RR 0.96, 95% CI 0.81 to 1.15, two studies, 1228 women; four or one visits versus none RR 0.97, 95% CI 0.80 to 1.17, one study, 876 women; and, four visits versus one RR 0.90, 95% CI 0.52 to 1.54, one study, 352 women) ( Analysis 1.3 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 3 Severe maternal morbidity.
Maternal health problems up to 42 days
Only one study reported results for most of our pre‐specified outcomes relating to maternal postpartum health problems up to 42 days after the birth ( Bashour 2008a ; Bashour 2008b ). There was no evidence of differences between women receiving four or one postnatal home visits versus none for secondary postpartum haemorrhage (RR 0.78, 95% CI 0.49 to 1.26); abdominal pain (RR 1.06, 95% CI 0.83 to 1.34); back pain (RR 0.96, 95% 0.83 to 1.11); urinary tract complications (RR 0.83, 95% CI 0.63 to 1.10); fever (RR 1.30, 95% CI 0.93 to 1.82) or dyspareunia (RR 1.18, 95% CI 0.90 to 1.55). No studies reported on thromboembolic disease or puerperal genital tract infections.
One study reported mean scores on a scale measuring maternal perceptions of their general health at six weeks postpartum ( Morrell 2000 ). There was no strong evidence of differences between women receiving additional postnatal support and controls (mean difference (MD) ‐1.60, 95% CI ‐4.72 to 1.52) ( Analysis 1.12 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 12 Maternal perception of general health at 6 weeks (mean SF36).
Postnatal depression and anxiety
None of the studies included in this comparison reported the number of women with a diagnosis of depression in the postnatal period. Two studies looked at mean scores on the Edinburgh postnatal depression scale (EPDS) at six weeks ( Morrell 2000 ) and eight weeks postpartum ( Christie 2011 ). In the Morrell 2000 study women received additional support from lay people, and in Christie 2011 , women received additional health visitor support as well as routine midwife home visits. The intervention did not appear to have a positive effect in either study, and overall, women receiving the additional visits had slightly higher mean depression scores (MD 1.05, 95% CI 0.27 to 1.82) ( Analysis 1.14 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 14 Mean postnatal depression score (last assessment up to 42 days postpartum).
Christie 2011 reported mean anxiety scores at eight weeks postpartum; there was no strong evidence of a difference between groups (MD 3.80, 95% CI ‐0.44 to 8.04) ( Analysis 1.16 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 16 Mean maternal anxiety score (last assessment up to 42 days postpartum).
Maternal satisfaction with care in the postnatal period
Women were asked about their satisfaction with postnatal care in two studies. In one study the number of women saying they were "happy" with their postnatal experience was reported ( Bashour 2008a ; Bashour 2008b ) ( Analysis 1.17 ). Women receiving no formal postnatal care were slightly more satisfied with their experience, but there was no evidence of difference between groups, and in all groups most women reported satisfaction (RR 0.96, 0.90 to 1.02). In a second study ( Christie 2011 ), the additional support provided by health visitors was associated with increased mean satisfaction scores (MD 14.70, 95% CI 8.32 to 21.08) ( Analysis 1.18 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 17 Maternal satisfaction with postnatal care.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 18 Mean satisfaction score with postnatal care.
Infant outcomes
Neonatal health service use.
Three studies reported the number of babies requiring urgent health care during the postnatal period although the way this outcome was defined varied in the three studies ( Bashour 2008a ; Bashour 2008b reported hospital visits up to four months; Ransjo‐Arvidson 1998 doctor‐identified infant health problem at six weeks; and Christie 2011 use of emergency medical services up to eight weeks). Overall, babies were less likely to have emergency medical care if their mothers received more postnatal home visits (average RR 0.65, 95% CI 0.45 to 0.95, three studies with 1370 infants) ( Analysis 1.19 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 19 Serious neonatal morbidity up to 6 months.
Breastfeeding
Exclusive breastfeeding at up to six weeks was reported in three studies. Women receiving additional support at home were more likely to be exclusively breastfeeding their babies at six weeks postpartum, and at the last assessment up to six months postpartum (average RR 1.17, 95% CI 1.01 to 1.36, three studies 960 women, and, average RR 1.38, 95% CI 1.10 to 1.73, three studies 1309 women respectively) ( Analysis 1.20 ; Analysis 1.21 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 20 Exclusive breastfeeding (last assessment up to 6 weeks).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 21 Exclusive breastfeeding (last assessment up to 6 months).
For any breastfeeding there was no evidence of differences between women receiving additional postnatal visits and controls at either six weeks or up to six months postpartum (average RR 0.89, 95% CI 0.57 to 1.38, two studies 813 women, and, average RR 1.01, 95% CI 0.99 to 1.03, two studies 1315 women, respectively) ( Analysis 1.22 ; Analysis 1.23 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 22 Any breastfeeding (up to 6 weeks).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 23 Any breastfeeding (last assessment up to 6 months).
Aksu 2010 reported mean duration of breastfeeding (months) in 54 women who had received one versus no postnatal care at home. In both groups women on average breast fed their babies for approximately a year or more, but the mean duration was increased by three months in women receiving a home visit (MD 3.00, 95% CI 2.33 to 3.67) ( Analysis 1.24 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 24 Mean duration of any breastfeeding (months).
Neonatal morbidity
Two studies reported infant respiratory tract infections up to eight weeks postpartum, although the condition was not defined in the same way in the two trials. In Bashour 2008a ; Bashour 2008b the number of babies suffering a cough or cold was reported, whereas in the Ransjo‐Arvidson 1998 trial infants appeared to have more serious illness. Overall, and in individual studies there was no clear evidence of difference between groups (pooled RR 0.99, 95% CI 0.84 to 1.17, two studies 1217 infants) ( Analysis 1.25 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 25 Infant respiratory tract infection within 42 days.
A single study reported on the number of infants with jaundice (not defined); very similar numbers of infants had jaundice in both intervention and control groups (RR 1.04, 95% CI 0.85 to 1.26, 861 infants) ( Analysis 1.26 ). In the same study, approximately half of the babies were reported to have had diarrhoea, however, more infants in the group receiving no visits were reported to suffer from diarrhoea compared to those whose mothers received postnatal home visits (RR 0.85, 95% CI 0.74 to 0.98, 861 infants) ( Analysis 1.27 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 26 Infant jaundice.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 27 Infant diarrhoea up to 42 days postpartum.
There were no clear differences between groups in the number of infants receiving immunisations; the vast majority of infants were immunised whether or not their mothers received postnatal care at home (RR 0.99, 95% CI 0.96 to 1.01) ( Analysis 1.28 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 28 Infant immunisation took place.
Non‐prespecified outcomes
One study reported on contraceptive use at 42 days postpartum; no clear differences between groups were identified (RR 0.98, 95% CI 0.82 to 1.16) ( Analysis 1.29 ).

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 29 Non prespecified ‐ Contraceptive use.
Comparison 2: Schedules comparing different models of postnatal care (three studies with 4394 women)
Three studies are included in this comparison; each examined a different type of intervention and control condition and we have not pooled findings in meta‐analyses. In brief, Steel 2003 compared two home visits compared with a telephone screening interview with discretionary nurse home visits. In Kronborg 2007 , health visitors (HVs) were randomised, and women were visited between one and three times by HVs who had attended special training on supporting breastfeeding compared with usual care by HVs who had been specially trained. MacArthur 2002 compared individualised postnatal care up to 10 to 12 weeks postpartum with usual care, which involved a more rigid schedule of midwife home visits in the early postnatal period.
For most of our prespecified outcomes no data were reported in any of the three trials.
None of the studies reported on maternal mortality.
In the study by MacArthur 2002 there were only three neonatal deaths from a sample of 2064 women, and no significant differences between treatment groups were identified.
None of the studies reported on maternal general morbidity although MacArthur 2002 reported on the number of women with EPDS scores greater than 12 (the cut‐off used to denote high risk of postnatal depression) at four months postpartum. Women receiving individualised extended postnatal care were less likely to have EPDS scores ≥ 13 compared with women receiving routine care (RR 0.68, 95% CI 0.53 to 0.86) ( Analysis 2.9 ).

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 9 Postnatal depression (EPDS ≥ 13 at 4 months postpartum).
Steel 2003 reported the number of babies with health problems up to four weeks; there was no strong evidence of any difference between groups (RR 0.97, 95% CI 0.85 to 1.12) ( Analysis 2.15 ).

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 15 Neonatal morbidity up to 28 days.
The cluster‐randomised trial by Kronborg 2007 examined the impact of care from HVs with special training to promote and support breastfeeding. In this study there was no evidence of difference in the number of women who had stopped exclusive breastfeeding at six weeks (RR 0.81, 95% CI 0.58 to 1.14) ( Analysis 2.16 ). Few women in either group continued to exclusively breastfeed at six months and there was no evidence of difference between groups identified (RR 1.47, 95% CI 0.81 to 2.69) ( Analysis 2.17 ).

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 16 Stopped exclusive breastfeeding (last assessment up to 6 weeks).

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 17 Exclusive breastfeeding (last assessment up to 6 months).
In the study comparing home visits versus telephone screening by Steel 2003 most women in both groups were breastfeeding their babies at six weeks postpartum (any breastfeeding) and there was no clear difference between groups (RR 1.03, 95% CI 0.99 to 1.08) ( Analysis 2.18 ).

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 18 Any breastfeeding (up to 6 weeks).
None of our other prespecified infant outcomes were reported in any of these studies.
Comparison 3: Home versus hospital postnatal care (four studies with 3917 women)
Four studies compared women attending hospital clinics for postnatal checks (usual care) versus home visits by nurses ( Escobar 2001 ; Gagnon 2002 ; Lieu 2000 ; Paul 2012 ).
None of these studies reported on maternal or neonatal mortality.
Maternal morbidity
All four studies reported on maternal use of emergency health care in the postnatal period although there were some differences in definitions; Escobar 2001 and Lieu 2000 reported on the number of women making an urgent hospital visit up to two weeks, and Paul 2012 the number of women seeking unplanned emergency health care up to two weeks, whereas Gagnon 2002 reported hospital admissions up to eight weeks postpartum. Pooled results from these studies revealed no evidence of differences between women receiving hospital clinic versus home postnatal care (RR 1.04, 95% CI 0.85 to 1.26, four studies, 3755 women) ( Analysis 3.3 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 3 Severe maternal morbidity.
Maternal anxiety and depression
Two studies reported on the number of women with depressive symptoms at two weeks postpartum; similar numbers of women in the intervention and control groups had symptoms (RR 1.10, 95% CI 0.93 to 1.30, two studies with 2177 women) ( Analysis 3.10 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 10 Postnatal depression (last assessment up to 42 days postpartum).
Gagnon 2002 reported mean scores on the State Trait Anxiety Inventory (STAI) at two weeks. There were no evidence of differences between groups (MD 0.30, 95% CI ‐1.08 to 1.68, 513 women) ( Analysis 3.13 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 13 Mean maternal anxiety score (last assessment up to 42 days postpartum).
Data on depression and anxiety were also collected in the Paul 2012 study. However, while the MDs between groups were set out, mean scores for women in the home and hospital groups were not reported and we were unable to enter data from this trial in our data and analyses tables. The authors reported no statistically significant differences in mean EPDS or STAI scores at two weeks, two months and six months postpartum.
Satisfaction with care
In two studies, women seemed to prefer home rather than hospital clinic care; postnatal care was rated as good or excellent by 68% of women in the home care group compared with 55% in the clinic group (unweighted percentages). This difference between groups was statistically significant (there was high heterogeneity for this outcome and we used a random‐effects model; average RR 1.26, 95% CI 1.09 to 1.45) ( Analysis 3.14 ). Gagnon 2002 identified no evidence of difference in mean scores for satisfaction with postnatal care at eight weeks (MD ‐0.10, 95% CI ‐0.88 to 0.68) ( Analysis 3.15 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 14 Maternal satisfaction with postnatal care.

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 15 Mean satisfaction score with postnatal care.
All four studies examined at least one outcome relating to breastfeeding. Gagnon 2002 reported the number of women exclusively breastfeeding at two weeks. There was no strong evidence of differences between groups (RR 1.05, 95% CI 0.93 to 1.18) ( Analysis 3.16 ). Escobar 2001 and Lieu 2000 reported the number of women who had discontinued any breastfeeding at two weeks; again, there were no clear differences between groups (RR 0.93, 95% CI 0.78 to 1.12) ( Analysis 3.18 ). Paul 2012 examined the number of women breastfeeding at eight weeks postpartum and while slightly more women in the home visit group were still breastfeeding at this time, the difference between groups did not reach statistical significance (RR 1.09, 95% CI 1.00 to 1.18) ( Analysis 3.19 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 16 Exclusive breastfeeding (last assessment up to 6 weeks).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 18 Discontinued breastfeeding (up to 6 weeks).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 19 Any breastfeeding (last assessment up to 6 months).
Infant severe morbidity and health care use
All four studies reported on infant use of emergency health care; Escobar 2001 and Lieu 2000 reported on the number of infants re‐hospitalised within two weeks of initial discharge, and Paul 2012 the number of infants requiring unplanned emergency health care up to two weeks. Gagnon 2002 reported infant hospital admissions up to eight weeks postpartum. Pooled results revealed no evidence of differences in infant health service use for women receiving hospital clinic versus home postnatal care (RR 1.11, 95% CI 0.86 to 1.43, four studies 3770 infants) ( Analysis 3.21 ).

Comparison 3 Postnatal visit at home vs hospital clinic visit, Outcome 21 Severe infant morbidity up to 6 weeks.
Planned subgroup and sensitivity analysis
For our primary outcomes we had planned subgroup analysis by when visits were initiated, by the duration and intensity of the intervention, by the person carrying out the visit (medical professional versus skilled attendant), by content of the visit, by parity, and by other potential modifying factors (e.g. study in developed versus developing country). However, within each comparison, data for our primary outcomes were scarce. For maternal mortality only one study reported findings and for neonatal mortality, within the different comparisons, at most two studies contributed data. For both of our primary outcomes event rates were low and within studies there were no significant differences between groups, nor was there evidence of heterogeneity between studies where more than one study contributed data. For these reasons, we did not think that in this version of the review subgroup analysis would throw any further light on findings. In future updates of the review, as more data become available we will carry out planned additional analysis.
Similarly, planned sensitivity analysis by risk of bias was not performed; again, too few studies contributed data to any particular analysis to make such additional analyses meaningful.
Summary of main results
In this review we have included data from 12 randomised trials with data for more than 11,000 women. The trials were carried out in countries across the world, and in both high‐ and low‐resource settings. In low‐resource settings, women receiving usual care may have received no additional postnatal care after early hospital discharge.
The interventions and control conditions varied considerably across studies with trials focusing on three broad types of comparisons: schedules involving more versus fewer postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus hospital clinic postnatal check‐ups (four studies). In all but two of the included studies, postnatal care at home was delivered by healthcare professionals. The broad aims of all interventions were to assess the wellbeing of mothers and babies, and to provide education and support, although some interventions had more specific aims such as to encourage breastfeeding or to provide practical support.
In the five studies comparing more versus less postnatal home visits there was no evidence of differences between groups for maternal and neonatal mortality. Only one study (which reported a large number of outcomes overall) reported results for most of our outcomes relating to maternal morbidity, and there was no strong evidence that more postnatal visits at home were associated with improvements in maternal health.
Two studies examining maternal depression compared mean scores on the EPDS and results suggested that women receiving more visits had higher mean scores, denoting an increased risk of depression. The reason for this finding is not clear. It is possible that women who had more contact with healthcare professionals may have been more willing to disclose their feelings. The authors of one trial ( Morrell 2000 ), also speculated that increased provision of support may somehow disrupt women's usual support networks, or that the withdrawal of services may result in increased depression.
Two studies reported on maternal satisfaction with postnatal care and in one of these, additional health visitor support was associated with increased satisfaction scores. There was some evidence that postnatal care at home may reduce infant health service utilisation in the weeks following the birth, and that more home visits may encourage more women to exclusively breastfeed their babies. The evidence regarding any breastfeeding was less clear, although one study with a small sample size suggested that a home visit may encourage women to continue to breastfeed for longer. There was no strong evidence that infant morbidity including jaundice and respiratory tract infections was affected by home visits, although episodes of diarrhoea were reported less often by women in the groups receiving visits in a single study (this study reported a large number of outcomes and as findings were not consistent it is possible that this finding occurred by chance).
For the three studies comparing different ways of offering care involving postnatal home visits it was not clear that interventions had a consistent effect, and many of our prespecified outcomes were not reported. There did not appear to be strong evidence from two studies that experimental interventions increased the number of women breastfeeding their babies. In one study, women in the experimental groups receiving an extended programme of home visits by midwives appeared to have lower EPDS scores at four months postpartum.
Four studies examined home versus hospital clinic postnatal checks. There were no data reported for most of our outcomes. There were no clear differences between groups for maternal emergency healthcare utilisation or maternal anxiety or depression. In two studies women seemed to prefer home rather than hospital care, while a third study examining satisfaction with care did not identify any clear difference between groups. There was no strong evidence that home care was associated with an increase in breastfeeding, or that infant emergency healthcare utilisation differed between groups.
Overall completeness and applicability of evidence
The studies included in the review examined different sorts of interventions in different types of settings and drawing clear conclusions is not simple. The trials had a variety of aims with some focusing on physical checks of the mother and newborn, while others specifically aimed to provide support for breastfeeding, and one included the provision of more practical support with housework and childcare; under these circumstances it is not surprising that results from studies were not entirely consistent. This variation in aims was reflected in the choice of outcomes reported in different studies, and for most of our outcomes there were very few data. Further, for outcomes such as breastfeeding there were differences in how outcomes were measured and when. Important clinical outcomes relating to maternal and infant health were mainly not reported, and for these outcomes results were dominated by a single study. Perhaps surprisingly, not all of the studies reported maternal satisfaction with different schedules or ways of offering care; those studies that did, provided some evidence that women preferred care at home. Improved maternal satisfaction with care involving home visits may be related to women's increased health awareness, support for behavioural change, and improved access to health‐care services, however, the evidence on maternal views is still limited. There was some evidence from two studies carried out in high‐resource settings that maternal depression scores were increased in women receiving more postnatal visits; the reasons for this finding are not clear, and this finding warrants further research attention in future trials and qualitative research.
Quality of the evidence
The studies included in the review were of mixed quality as regards risk of bias. Most of the studies used methods to generate the randomisation sequence and to conceal allocation at the point of randomisation that we judged were at low risk of bias. On the other hand, blinding women and healthcare staff to this type of intervention is not generally feasible, and in many of the studies the experimental and control interventions may have been delivered by quite different staff. Although in eight of the 12 studies it was stated that outcome assessors were blind to treatment group, many of the data on breastfeeding and maternal depression, for example, were derived from interviews and it is possible that women disclosed their allocation. It is also possible that the interventions themselves led to a response bias; women in the groups receiving more care were asked to discuss their health (physical and psychological) as part of the intervention, and it is possible that this may have affected reporting of health problems as part of study assessments. Loss to follow‐up was a further problem in half of these trials. Even relatively low sample attrition (less than 5%) may mean it is more difficult to interpret results for outcomes that occur infrequently (such as serious maternal morbidity) as those with health problems may be less likely to respond. We were unable to investigate possible publication bias as too few studies contributed data.
Most of the results in the review are derived from one or two studies and several of the studies had small sample sizes; we were unable to pool many of the data in meta‐analysis; there was a lack of consistency between studies in terms of the outcomes reported, and the time and way in which outcomes were measured. In addition, there was considerably diversity in terms of the aims of interventions and the way they were delivered. These differences mean that for any one outcome there were few data and most of our results were inconclusive.
Potential biases in the review process
We are aware that authors carrying out a review may themselves introduce bias. We took a number of measures to try to reduce bias; at least two review authors carried out data extraction and assessed risk of bias. All data were checked after entry. Nevertheless, assessing risk of bias for example, requires individual judgements and it is possible that a different review team may have made different assessments.
Agreements and disagreements with other studies or reviews
Generally, postnatal home visits seem likely to increase maternal satisfaction, and may promote breastfeeding, and reduce infant morbidities, but these effects are very much dependent upon the aims of the package of the postnatal interventions. The findings are in line with what the previous literature has shown.
Implications for practice
Generally, postnatal home visits have been recommended where mothers and their newborns are discharged early to promote infant health and maternal satisfaction. However, the results of this review are inconclusive and in the absence of strong or consistent evidence the frequency, timing, duration and intensity of such postnatal care visits should be determined by individual and local needs, and where possible, should take account of maternal preferences.
Implications for research
Further well designed randomised controlled trials or any other studies evaluating this complex intervention will be required to formulate the optimal package. The design of interventions in such a trial should be based upon postpartum health priorities in each context, which would determine the intensity and content of postnatal care visits.
Feedback from MacArthur and Bick, 12 March 2015
The findings of our study ( MacArthur 2002 ) have been included in this review in the opposite direction to the results reported in our Lancet paper. The review states that the intervention group had worse (higher) EPDS scores than the control group, which is opposite to the actual findings.
The review concludes "Significantly more women receiving extended postnatal care had high EPDS scores (RR 1.47, 95% CI 1.13 to 1.92) (Analysis 2.9)". This is completely wrong as it was significantly FEWER, not more.
Similarly, the Discussion states “For the three studies comparing different ways of offering care involving postnatal home visits it was not clear that interventions had a consistent or positive effect, and many of our prespecified outcomes were not reported. There did not appear to be strong evidence from two studies that experimental interventions increased the number of women breastfeeding their babies. In one study, women in the experimental groups receiving an extended programme of home visits by health visitors appeared to have higher EPDS scores. The reason for this finding is not clear". Again this is incorrect.
This serious inaccuracy should be rectified, and the conclusions of the overall review amended.
Comment submitted by Christine MacArthur and Debra Bick, March 2015
Thanks to Professors MacArthur and Bick for this feedback. The feedback is correct ‐ in a previous version of this review data on postnatal depression scores for the MacArthur 2002 trial was entered the wrong way around and appeared to favour the control group. The review team apologises for this serious data entry mistake and the data have now been corrected. The text has also been amended in the abstract, the plain language summary, the main results section and the discussion, so that it is now clear that results from this trial show a reduction in depression scores in the group receiving an extended programme of home visits by health visitors.
Contributors
Reply from Naohiro Yonemo, Therese Dowswell, Shuko Nagai, and Rintaro Mori, April 2017
Protocol first published: Issue 9, 2011 Review first published: Issue 7, 2013
Acknowledgements
The authors would like to acknowledge the help received from the Cochrane Pregnancy and Childbirth Group and Thai Cochrane Network.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Edited (no change to conclusions), comment added to review
Data and analyses
Comparison 1.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 4 Secondary postpartum haemorrhage.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 5 Abdominal pain up to 42 days postpartum.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 6 Back pain up to 42 days postpartum.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 9 Urinary tract complications up to 42 days postpartum.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 10 Maternal fever up to 42 days postpartum.

Comparison 1 Schedules involving more vs fewer postpartum visits, Outcome 11 Dyspareunia.
Comparison 2

Comparison 2 Studies comparing different ways of offering postnatal care at home, Outcome 2 Neonatal mortality.
Comparison 3
Characteristics of studies, characteristics of included studies [ordered by study id].
EPDS: Edinburgh Postnatal Depression Scale ITT: intention to treat MCS: mental health score PCS: physical health score NA: not applicable RhoGAM: Rh (D) immunoglobulin SF‐36: short 36 STAI: State‐Trait Anxiety Inventory vs: versus
Characteristics of excluded studies [ordered by study ID]
Characteristics of studies awaiting assessment [ordered by study id], differences between protocol and review.
In the objectives and types of interventions sections, we changed the description of interventions. The interventions and control conditions varied considerably across studies with trials focusing on three broad types of comparisons: schedules involving more versus fewer postnatal home visits, schedules involving different models of care, and home versus hospital clinic postnatal check‐ups.
Contributions of authors
Naohiro Yonemoto, Shuko Nagai and Rintaro Mori contributed to conceptualisation of this review and development of the protocol. Naohiro Yonemoto and Shuko Nagai screened and reviewed the identified studies, and contributed to data entry. Naohiro Yonemoto contributed to the analyses. Therese Dowswell also reviewed the identified studies and contributed to analyses and preparation of the manuscript. Rintaro Mori also contributed to writing and advised on the analyses. All the review authors approved the final version of the review.
Sources of support
Internal sources.
- National Centre of Neurology and Psychiatry, Japan.
External sources
- Health and Labour Sciences Research Grants, Japan.
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS 10/4001/02
Declarations of interest
None known.
References to studies included in this review
Aksu 2010 {published data only}.
- Aksu H, Kucuk M, Duzgun G. The effect of postnatal breastfeeding education/support offered at home 3 days after delivery on breastfeeding duration and knowledge: a randomized trial . Journal of Maternal‐Fetal and Neonatal Medicine 2011; 24 ( 2 ):354‐61. [ PubMed ] [ Google Scholar ]
Bashour 2008a {published data only}
- Bashour HN, Kharouf MH, Abdulsalam AA, Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial . Public Health Nursing 2008; 25 ( 2 ):115‐25. [ PubMed ] [ Google Scholar ]
Bashour 2008b {published data only}
Christie 2011 {published data only}.
- Christie J, Bunting B. The effect of health visitors' postpartum home visit frequency on first‐time mothers: cluster randomised trial . International Journal of Nursing Studies 2011; 48 ( 6 ):689‐702. [ PubMed ] [ Google Scholar ]
Escobar 2001 {published data only}
- Escobar G, Braveman P, Ackerson L, Odouli R, Coleman‐Phox K, Lieu T. Home visits vs. hospital‐based group follow‐up visits after early postpartum discharge [abstract] . Pediatric Academic Societies Annual Meeting; 2001 April 28‐May 1; Baltimore, USA . 2001:Abstract no: 1111. [ PubMed ]
- Escobar GJ, Braveman PA, Ackerson L, Odouli R, Coleman‐Phox K, Capra AM, et al. A randomized comparison of home visits and hospital‐based group follow‐up visits after early postpartum discharge . Pediatrics 2001; 108 :719‐27. [ PubMed ] [ Google Scholar ]
Gagnon 2002 {published data only}
- Gagnon AJ, Dougherty G, Jimenez V, Leduc N. Randomized trial of postpartum care after hospital discharge . Pediatrics 2002; 109 ( 6 ):1074‐80. [ PubMed ] [ Google Scholar ]
Kronborg 2007 {published data only}
- Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster‐randomized community based trial . Acta Paediatrica 2007; 96 ( 7 ):1064‐70. [ PubMed ] [ Google Scholar ]
Lieu 2000 {published data only}
- Lieu TA, Braveman PA, Escobar GJ, Fischer AF, Jensvold NG, Capra AM. A randomized comparison of home and clinic follow‐up visits after early postpartum hospital discharge . Pediatrics 2000; 105 :1058‐65. [ PubMed ] [ Google Scholar ]
MacArthur 2002 {published data only}
- MacArthur C, Winter HR, Bick DE, Knowles H, Lilford R, Henderson C, et al. Effects of redesigned community postnatal care on womens' health 4 months after birth: a cluster randomised trial . Lancet 2002; 359 :378‐85. [ PubMed ] [ Google Scholar ]
Morrell 2000 {published data only}
- Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and effectiveness of community postnatal support workers: randomised controlled trial . BMJ 2000; 321(7261) :593‐8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Paul 2012 {published data only}
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA, et al. A randomized trial of single home nursing visits vs office‐based care after nursery/maternity discharge: the Nurses for Infants Through Teaching and Assessment After the Nursery (NITTANY) Study . Archives of Pediatrics & Adolescent Medicine 2012; 166 ( 3 ):263‐70. [ PubMed ] [ Google Scholar ]
Ransjo‐Arvidson 1998 {published data only}
- Ransjo‐Arvidson AB, Chintu K, Ng'andu N, Eriksson B, Susu B, Christensson K, et al. Maternal and infant health problems after normal childbirth: a randomised controlled study in Zambia . Journal of Epidemiology & Community Health 1998; 52 :385‐91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Steel 2003 {published data only}
- Steel O'Connor KO, Mowat DL, Scott HM, Carr PA, Dorland JL, Young Tai KF. A randomized trial of two public health nurse follow‐up programs after early obstetrical discharge: an examination of breastfeeding rates, maternal confidence and utilization and costs of health services . Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2003; 94 ( 2 ):98‐103. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Young Tai KFW, O'Connor KO, Scott HM, Mowat DL, Carr PA. Do community follow‐up programs improve infant outcome after early obstetrical discharge? A randomized controlled trial. [abstract] . Pediatric Research 2000; 47 ( 4 ):161A. [ Google Scholar ]
References to studies excluded from this review
Boulvain 2004 {published data only}.
- Boulvain M, Perneger TV, Othenin‐Girard V, Petrou S, Berner M, Irion O. Home‐based versus hospital‐based postnatal care: a randomised trial . BJOG: an international journal of obstetrics and gynaecology 2004; 111 ( 8 ):807‐13. [ PubMed ] [ Google Scholar ]
Carty 1990 {published data only}
- Carty EM, Bradley CF. A randomized, controlled evaluation of early postpartum hospital discharge . Birth 1990; 17 :199‐204. [ PubMed ] [ Google Scholar ]
Gunn 1998 {published data only}
- Gunn J, Lumley J, Chondros P, Young D. Does an early postnatal check‐up improve maternal health: results from a randomised trial in Australian general practice . British Journal of Obstetrics and Gynaecology 1998; 105 ( 9 ):991‐7. [ PubMed ] [ Google Scholar ]
Izzo 2005 {published data only}
- Izzo CV, Eckenrode JJ, Smith EG, Henderson CR, Cole R, Kitzman H, et al. Reducing the impact of uncontrollable stressful life events through a program of nurse home visitation for new parents . Prevention Science 2005; 6 ( 4 ):269‐74. [ PubMed ] [ Google Scholar ]
Korfmacher 1999 {published data only}
- Korfmacher J, O'Brien R, Hiatt S, Olds D. Differences in program implementation between nurses and paraprofessionals providing home visits during pregnancy and infancy: a randomized trial . American Journal of Public Health 1999; 89 ( 12 ):1847‐51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Lumley 2006 {published data only}
- Lumley J, Small R, Brown S, Watson L, Gunn J, Mitchell C, et al. Prism (program of resources, information and support for mothers) protocol for a community‐randomised trial [isrctn03464021] . BMC Public Health 2003; 3 :36. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lumley J, Watson L, Small R, Brown S, Mitchell C, Gunn J. Prism (program of resources, information and support for mothers): a community‐randomised trial to reduce depression and improve women's physical health six months after birth . BMC Public Health 2006; 6 :37. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Willis K, Small R, Brown S. Using documents to investigate links between implementation and sustainability in a complex community intervention: the PRISM study . Social Science & Medicine 2012; 75 ( 7 ):1222‐9. [ PubMed ] [ Google Scholar ]
Olds 2002 {published data only}
- Olds DL, Robinson J, O'Brien R, Luckey DW, Pettitt LM, Henderson CR Jr, et al. Home visiting by paraprofessionals and by nurses: a randomized, controlled trial. [Comment] . Pediatrics 2002; 110 ( 3 ):486‐96. [ PubMed ] [ Google Scholar ]
Quinlivan 2003 {published data only}
- Quinlivan JA, Box H, Evans SF. Postnatal home visits in teenage mothers: a randomised controlled trial . Lancet 2003; 361 ( 9361 ):893‐900. [ PubMed ] [ Google Scholar ]
Simons 2001 {published data only}
- Simons J, Reynolds J, Morison L. Randomised controlled trial of training health visitors to identify and help couples with relationship problems following a birth . British Journal of General Practice 2001; 51 :793‐9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Stanwick 1982 {published data only}
- Stanwick RS, Moffat MEK, Robitaille Y, Edmond A, Dok C. An evaluation of the routine postnatal public health nurse home visit . Canadian Journal of Public Health 1982; 73 :200‐5. [ PubMed ] [ Google Scholar ]
References to studies awaiting assessment
Furnieles‐paterna 2011 {published data only}.
- Furnieles‐Paterna E, Hoyuelos‐Camara H, Montiano‐Ruiz I, Penalver‐Julve N, Fitera‐Lamas L. Randomized comparative study of the puerperal visits in the mothers house and in the health center [Spanish] [Estudio comparativo y aleatorizado de la visita puerperal en el domicilio de la madre y en el centro de salud]. Matronas Profesion 2011; 12 ( 3 ):65‐73. [ Google Scholar ]
Salazar 2011 {published data only}
- Salazar I, Sainz JA, Garcia E, Marrugal V, Garrido R. Influence of early postpartum home visits on the detection and clinical course of postpartum depression . Progresos de Obstetricia y Ginecologia 2011; 54 ( 2 ):65‐70. [ Google Scholar ]
Additional references
- American Academy of Pediatrics. Council on Child and Adolescent Health. The role of home‐visitation programs in improving health outcomes for children and families . Pediatrics 1998; 101 ( 3 ):486‐9. [ PubMed ] [ Google Scholar ]
- American Academy of Pediatrics. Council on Child and Adolescent Health. The role of preschool home‐visiting programs in improving children's developmental and health outcomes . Pediatrics 2009; 123 ( 2 ):598‐603. [ PubMed ] [ Google Scholar ]
Barlow 2006
- Barlow J, Davis H, McIntosh E, Jarrett P, Mockford C, Stewart‐Brown S. Role of home visiting in improving parenting and health in families at risk of abuse and neglect: results of a multicentre randomised controlled trial and economic evaluation . Archives of Disease in Childhood 2007; 92 ( 3 ):229‐33. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Bashour 2008
Bennett 2008.
- Bennett C, Macdonald G, Dennis JA, Coren E, Patterson J, Astin M, et al. Home‐based support for disadvantaged adult mothers . Cochrane Database of Systematic Reviews 2008, Issue 1 . [DOI: 10.1002/14651858.CD003759.pub3] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown S, Small R, Faber B, Krastev A, Davis P. Early postnatal discharge from hospital for healthy mothers and term infants . Cochrane Database of Systematic Reviews 2002, Issue 3 . [DOI: 10.1002/14651858.CD002958] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Carabin 2005
- Carabin H, Cowan LD, Beebe LA, Skaggs VJ, Thompson D, Agbangla C. Does participation in a nurse visitation programme reduce the frequency of adverse perinatal outcomes in first‐time mothers? . Paediatric and Perinatal Epidemiology 2005; 19 ( 3 ):194‐205. [ PubMed ] [ Google Scholar ]
Doggett 2005
- Doggett C, Burrett S, Osborn DA. Home visits during pregnancy and after birth for women with an alcohol or drug problem . Cochrane Database of Systematic Reviews 2005, Issue 4 . [DOI: 10.1002/14651858.CD004456.pub2] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Donovan 2007
- Donovan EF, Ammerman RT, Besl J, Atherton H, Khoury JC, Altaye M, et al. Intensive home visiting is associated with decreased risk of infant death . Pediatrics 2007; 119 ( 6 ):1145‐51. [ PubMed ] [ Google Scholar ]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 . Available from www.cochrane‐handbook.org .
Jahanfar 2013
- Jahanfar S, Janssen PA, Howard LM, Dowswell T. Interventions for preventing or reducing domestic violence against pregnant women . Cochrane Database of Systematic Reviews 2013, Issue 2 . [DOI: 10.1002/14651858.CD009414.pub2] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lassi ZS, Haider BA, Bhutta ZA. Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes . Cochrane Database of Systematic Reviews 2010, Issue 11 . [DOI: 10.1002/14651858.CD007754.pub2] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Macdonald 2008
- Macdonald G, Bennett C, Dennis JA, Coren E, Patterson J, Astin M, et al. Home‐based support for disadvantaged teenage mothers . Cochrane Database of Systematic Reviews 2008, Issue 1 . [DOI: 10.1002/14651858.CD006723.pub2] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
McIntosh 2009
- McIntosh E, Barlow J, Davis H, Stewart‐Brown S. Economic evaluation of an intensive home visiting programme for vulnerable families: a cost‐effectiveness analysis of a public health intervention . Journal of Public Health 2009; 31 ( 3 ):423‐33. [ PubMed ] [ Google Scholar ]
- Olds DL, Eckenrode J, Henderson CR Jr, Kitzman H, Powers J, Cole R, et al. Long‐term effects of home visitation on maternal life course and child abuse and neglect. Fifteen‐year follow‐up of a randomized trial . JAMA 1997; 278 ( 8 ):637‐43. [ PubMed ] [ Google Scholar ]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) . Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Shaw E, Levitt C, Wong S, Kaczorowski J, McMaster University Postpartum Research Group. Systematic review of the literature on postpartum care: effectiveness of postpartum support to improve maternal parenting, mental health, quality of life, and physical health . Birth 2006; 33 ( 3 ):210‐20. [ PubMed ] [ Google Scholar ]
Turnbull 2012
- Turnbull C, Osborn DA. Home visits during pregnancy and after birth for women with an alcohol or drug problem . Cochrane Database of Systematic Reviews 2012, Issue 1 . [DOI: 10.1002/14651858.CD004456.pub3] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization. Make every mother and child count . The World Health Report 2005.
WHO/UNICEF 2009
- WHO/UNICEF. Joint statement. Home visits for the newborn child: a strategy to improve survival . WHO/FCH/CAH/09.02 2009. [ PubMed ]
References to other published versions of this review
Yonemoto 2011.
- Yonemoto N, Nagai S, Mori R. Schedules for home visits in the early postpartum period . Cochrane Database of Systematic Reviews 2011, Issue 9 . [DOI: 10.1002/14651858.CD009326] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
COMMENTS
Public Health Postnatal Services. Public health nurses provide support and health assessments to mothers, newborns and their families. Public health nurses contact families within 48 hours after discharge from the hospital. Services are offered according to the situation and discussion with the family and may include health assessments of the ...
If you gave birth at home, you may need to request a package of forms to register your baby's birth. Contact the Vital Statistics Office. In Edmonton, call toll-free at 780-427-7013. Elsewhere in Alberta, call toll-free at 310-000, then dial 780-427-7013. Birth certificate.
Welcome to Alberta Holistic Midwives. ... Approximately 3-4 home visits will be done in the first week postpartum, depending on individual needs. ... The home visits after my birth were fantastic and I couldn't have imagined it any other way. If any friends ask for midwives I will forever send them to Mary and Cher. I highly recommend!"
In the first few weeks, your baby will have 3 small poops or 1 large poop a day. After 1 month, your baby could poop once every few days and up to once a week. If you are formula feeding: Your baby's poop will be pale yellow to light brown, pasty or thick. In the first few weeks, your baby will poop 1 to 2 times a day.
Similarly, there was no difference between the two group's breastfeeding rates at six months. But the cost difference between the two interventions was significant, with the cost of the call group averaging about $150 per infant and the two-visit group averaging $240 per infant. Geary points out, however, that if the public health ...
Put a thin cloth between the ice and your skin. Also try sitting in 8 to 10 centimetres (3 to 4 inches) of warm water (sitz bath) 3 times a day and after bowel movements. Take pain medicines exactly as directed. If the doctor or midwife gave you a prescription medicine for pain, take it as prescribed.
3.3 Care of the Newborn. During the early postpartum period, care of the newborn usually involves celebrating and rejoicing with the family and respecting and supporting their needs. The care is based on nurturing the developing mother-baby-family relationship and caring for mother and baby as a unit.
Be referred by the Public Health nurse who assesses each new mother at the hospital to determine whether she can receive postnatal services from Public Health or other organizations. Call Public Health . Referral from a professional or a family member or friend. Mothers can be visited by a nurse and a dietitian at home before and after birth.
Bringing home a new baby can be one of the most exciting and stressful times in your life. A nurse might visit a couple of times, then other than routine check-ups at the nurse's office, you ...
Caring for yourself. Trust yourself. If something doesn't feel right with your body, tell your doctor right away. Sleep when your baby sleeps, drink plenty of water, and ask for help if you need it. Tell your doctor if you or your partner feels sad or anxious for more than 2 weeks. Call your doctor or midwife with questions about breastfeeding ...
More versus fewer home visits (five studies, 2102 women) ... In a cluster-RCT comparing usual care with individualised care by midwives, extended up to three months after the birth, there may be little or no difference in neonatal mortality (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 infants). The proportion of women with EPDS scores ≥ 13 ...
If parents decide to participate—previous studies show that more than 70 percent agree to join—the nurse schedules an in-home visit about three weeks after birth. 14 This initial visit lasts ...
home visits should be initiated as soon as possible after birth or after returning home from the facility… Additional visits on day 3 and, if possible, on day 7 can improve home care practices and identify danger signs or illness. Home visits can be done by health professionals or by appropriately-trained community health workers."
Coverage for postnatal home visitation within 2 days of birth is probably considerably lower than 16%. In Ghana, for the 27% of newborns born at home, PNC coverage within 4-48 hours of birth was 10%. Only 0.2% of newborns born at home were reported to have received ≤48hr PNC from community health officers or community health nurses, the two ...
Context and Policy Issues. In 2019, there were 372,329 babies born in Canada. 1 Public health early child home visiting programs have been delivered for many years in all provinces and territories in Canada. 2 Home visiting is a method for delivering a broad range of child development enhancement services to parents, newborns and their families. 3 It has the advantage of the individually ...
The primary objective of this review is to assess the effects of different home‐visiting schedules on maternal and newborn mortality during the early postpartum period. The review focuses on the frequency of home visits (how many home visits in total), the timing (when visits started, e.g. within 48 hours of the birth), duration (when visits ...
Welcome Baby provides a home visit before the 27th week of pregnancy, followed by a phone call check-in and a home visit after the 28th week of pregnancy. Families receive five home visits after their baby is born. A registered nurse makes the first visit 3 to 14 days postpartum. A parent coach then visits the family at 2 to 4 weeks, 2 months ...
Family Connects was implemented by 129 full-time equivalent (FTE) home visitors in 2022. Home visiting providers are Registered Nurses with up-to-date professional licenses, who function within the state's Nurse Practice Act and are trained to provide newborn, caregiver, and family health and psychosocial assessments.
The Home Visit. First things first, when the nurse arrives at your house for you home visit, she is not looking for a sparkling clean house. She is looking for a Mama resting. ... Home visits are completed between 36 and 72 hours after birth. The nurse will do an assessment on both mom and baby. For mom, this includes things such as vital signs ...
Shupe struggled to breastfeed in the first few weeks of Jaxxon's birth, but she found success with help from the Postpartum Home Visit Program. Now Jaxxon is a healthy 2-month-old. Photo by Joel Blocker, for UCHealth. The benefits of an at-home visit from a UCHealth nurse. The nurses like to do a home visit when the baby has been home for ...
The programme includes home visits from a family nurse while you're pregnant, and after your baby's born. These visits help: to have a healthy pregnancy. you and your baby grow and develop together. you to be the best parent you can be. Your health visitor will take over from your family nurse when your baby is two until they go to school.
There were no program effects on substance abuseor depression. Nurse-visited women were more likely to be married from child age 2 through 18 (19.2% vs 14.8%, P = .04), and those with higher psychological resources had 4.64 fewer cumulative years rearing subsequent children after the birth of the first child (P = .03). Pregnancy planning was a ...
The Nursing Homes Operations Regulation said each resident of a facility should get a minimum 1.9 hours of nursing and personal care each day, but the new Continuing Care Act Regulations are ...
There was no fixed schedule or number of postnatal visits. The number and content of visits at home was determined by midwives in consultation with women. After the initial visit a symptoms checklist was used and visits could take place up to 10‐12 weeks. (Midwife records suggest the mean number of visits was 6).