Star Trek Channel
A continuing journey..

Prime Directive
Honor Blade
Star Boat Part2. “The Space Blob”
Star Boat – “The Space Blob” Part 1
“Interlude” by Avalon Universe
Starship Republic “Serpent of Yesterday”
https://www.youtube.com/watch?v=X1gvy_kewM4
The Archive
Intrepid “Duty of Care”
Main Intrepid page
Star Trek: Absolution “The Galaxy Just Got Smaller (Part II)” — Episode 2

Star Trek Natures Hunger: “Scorned at the Captain’s Table” — Season 7, Episode 1

Project: Potemkin “Third Watch” — S03-E
Visit the main Project: Potemkin page .

When does the final season of 'Star Trek: Discovery' come out? Release date, cast, where to watch

It's time for U.S.S. Discovery's final mission.
Paramount+'s hit TV series "Star Trek: Discovery" is returning for its fifth and final season this week and there is a lot to look forward to.
"The fifth and final season will find Captain Burnham and the crew of the U.S.S. Discovery uncovering a mystery that will send them on an epic adventure across the galaxy to find an ancient power whose very existence has been deliberately hidden for centuries," says Paramount+ about the upcoming season. "But there are others on the hunt as well…dangerous foes who are desperate to claim the prize for themselves and will stop at nothing to get it."
"Star Trek: Discovery" debuted in 2017 and is the seventh in the Star Trek series. Here's everything you need to know about the final season of the series.
When does 'Star Trek: Discovery' Season 5 premiere?
The finale season of "Star Trek: Discovery" is scheduled to premiere on Paramount+ on Thursday, April 4.
The first two episodes will be available to stream on the premiere date, with new episodes dropping weekly on Thursdays. Paramount+ did not specify what time the episodes will be available on their platform.
'Star Trek: Discovery' on Paramount+: Subscribe
Kenneth Mitchell: 'Star Trek: Discovery' actor, dies after battle with ALS
'Star Trek: Discovery' Season 5 episodes
Season 5 of "Star Trek: Discovery" has 10 episodes in total. The first two will be available to stream on April 4, with the remaining dropping weekly on Thursday on Paramount+.
'Star Trek: Discovery' Season 5 cast
Season 5 of "Star Trek: Discovery" brings back new and old faces along with recurring guest stars. Cast members include:
- Sonequa Martin-Green as Captain Michael Burnham
- Doug Jones as Saru
- Anthony Rapp as Paul Stamets
- Mary Wiseman as Sylvia Tilly
- Wilson Cruz as Dr. Hugh Culber
- David Ajala as Cleveland “Book” Booker
- Blu del Barrio as Adira
- Callum Keith Rennie as Rayner.
- Elias Toufexis as L’ak
- Eve Harlow as Moll
'Star Trek: Discovery' Season 5 trailer
Paramount+ dropped the official trailer for Season 5 on Feb. 23.
Saman Shafiq is a trending news reporter for USA TODAY. Reach her at [email protected] and follow her on X, the platform formerly known as Twitter @saman_shafiq7.
- Where to watch in the US
- Where to watch in Canada
- Where to watch in New Zealand
- How to watch from anywhere
- How to watch with a VPN
Where to watch Star Trek: Discovery free — Final season starts today
When you buy through our links, Business Insider may earn an affiliate commission. Learn more
The newest season of Star Trek: Discovery is officially underway. Season 5 marks the final season of the Star Trek spin-off, and it's shaping up to be an action-packed swang song. Whether you're looking to stream the new episodes or get caught up on the past four seasons, we've got everything you need to know about the show, including where to watch Star Trek: Discovery free via a TV channel abroad.
Star Trek: Discovery premiered in 2017 and follows in the decades-long tradition of Star Trek stories. The series is set about five years before the original Star Trek, which chronicled Captain Kirk's five-year journey. In Star Trek: Discovery, the U.S.S. Discovery travels through space on a mission of exploration. Season 5 sees Captain Burnham (Sonequa Martin-Green) and the U.S.S. Discovery crew on the hunt for an ancient power that others are also seeking.
The first two premiere episodes are currently streaming. Keep reading to learn how to watch the series no matter where you are in the world.
- Where to watch American Horror Story | Where to watch 9-1-1 | Where to watch Game of Thrones
Where to watch Star Trek: Discovery in the US
New Season 5 episodes of Star Trek: Discovery land on Paramount+ on Thursdays. The premiere week includes two episodes, and then one new episode will drop weekly after that. Episodes should be available starting at about 3 a.m. ET. All four past seasons are available to stream through the service. Subscriptions start at $5.99 a month and come with a one-week free trial.
Paramount Plus' Essential tier is a steal at this price and only has limited ads. It features tons of on-demand content from Paramount, CBS, Nickelodeon, Comedy Central, BET, and MTV. And you get NFL and Champions League soccer live streaming. There's a 7-day free trial, then it's $6 a month or $60 a year. The only way to ditch the ads is by opting for the Showtime bundle.
Where to watch Star Trek: Discovery in Canada
Paramount+ is also the home to Star Trek: Discovery in Canada. Plans start at CAD$6.99 and come with a one-week free trial. All episodes are available to stream here.
Where to watch Star Trek: Discovery in New Zealand
Star Trek: Discovery is available to stream for free on TVNZ+ . You'll need to create a free account to start streaming. In addition to new season 5 episodes, Seasons 1-4 are also streaming on the site. New episodes are available on Thursdays.
How to watch Star Trek: Discovery from anywhere
If you're not in New Zealand at the moment, you can access streams with a VPN (virtual private network). VPNs alter your electronic device's location so you can use websites that might not be available in certain regions. They're also solid ways to boost your online privacy. We recommend ExpressVPN , a user-friendly option with a 30-day money-back guarantee. Check out our ExpressVPN review for additional details and see below to learn how to use a VPN.
With its consistent performance, reliable security, and expansive global streaming features, ExpressVPN is the best VPN out there, excelling in every spec and offering many advanced features that makes it exceptional. Better yet, you can save up to 49% and get an extra three months for free today.
How to watch Star Trek: Discovery with a VPN
- Sign up for a VPN if you don't have one.
- Install it on the device you're using to watch Star Trek: Discovery.
- Turn it on and set it to New Zealand.
- Go to TVNZ+ and create a log-in profile.
- Watch Star Trek: Discovery.
Note: The use of VPNs is illegal in certain countries, and using VPNs to access region-locked streaming content might constitute a breach of the terms of use for certain services. Insider does not endorse or condone the illegal use of VPNs.
You can purchase logo and accolade licensing to this story here . Disclosure: Written and researched by the Insider Reviews team. We highlight products and services you might find interesting. If you buy them, we may get a small share of the revenue from the sale from our partners. We may receive products free of charge from manufacturers to test. This does not drive our decision as to whether or not a product is featured or recommended. We operate independently from our advertising team. We welcome your feedback. Email us at [email protected] .

- Main content

- April 6, 2024 | ‘Star Trek: Discovery’ Showrunner Explains Why They Reopened A TNG Mystery To Start Season 5
- April 5, 2024 | Roddenberry Archive Expands With Virtual Tours Of Deep Space 9 Station And The USS Discovery
- April 5, 2024 | Podcast: All Access Reviews The First Two Episodes Of ‘Star Trek: Discovery’ Season 5
- April 4, 2024 | Recap/Review: ‘Star Trek: Discovery’ Embraces Second Chances In “Under The Twin Moons”
- April 4, 2024 | Recap/Review: ‘Star Trek: Discovery’ Returns With New Vitality And A Lore-Fueled Quest In “Red Directive”
Pluto TV Adds Dedicated ‘Star Trek: Deep Space Nine’ Channel

| April 2, 2024 | By: TrekMovie.com Staff 26 comments so far
PlutoTV is part of the Paramount Global portfolio of services, and as we’ve reported before , the ad-supported free streaming service has multiple Star Trek series that run on their “Star Trek” and “More Star Trek” channels. PlutoTV has been streaming Star Trek: The Original Series , The Next Generation , Deep Space Nine , and Voyager on those two live Star Trek channels. This week, PlutoTV launched a third channel in the USA, solely dedicated to a Deep Space Nine . This is a first for Trek on PlutoTV.
3 live Trek channels
Adding a channel just for DS9 is part of Pluto’s 10th anniversary celebration:
In April, we’re celebrating our 10th anniversary in a big way, welcoming a dedicated Deep Space Nine channel to our growing Star Trek lineup and more.
The Deep Space Nine channel is already up and running next to the two other Trek channels…
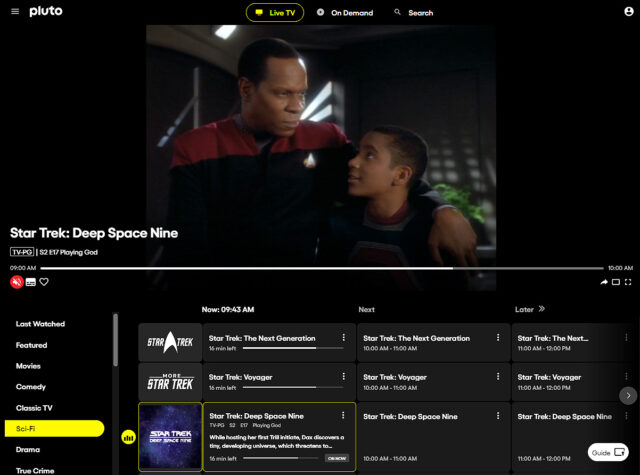
Pluto now has 3 Trek channels
Pluto’s original Star Trek channel is now dedicated to streaming episodes of TOS and TNG. For now the More Star Trek channel is streaming episodes of Voyager .
Trek on demand
In addition to episodes being shown on live-streaming channels, select seasons of Star Trek: The Original Series , Star Trek: The Next Generation , Star Trek: Deep Space Nine , and Star Trek: Voyager are also available on demand.
Star Trek plays a big part for Paramount’s free streamer. Periodically Pluto has Star Trek movies streaming live and on demand. And when new seasons of Paramount+ original Star Trek shows arrive they often use their “Paramount+ Picks” channel to stream season premiere episodes for free.
Pluto TV’s advertisements often feature Trek in some manner. This includes their most recent “Couch Potato” advert which aired during the Super Bowl.
And back in October, Pluto TV had this TNG-inspired advertisement
Pluto TV is available on the web at pluto.tv , and via apps for smart TVs, consoles, and mobile devices.
Keep up with the Star Trek Universe on TV here at TrekMovie.com .
Related Articles

Conventions/Events/Attractions , Fandom , Interview
How A Star Trek Drag Show Is Helping The Homeless In Oklahoma

Celebrity , DS9 , VOY
Armin Shimerman And Terry Farrell Join The ‘Delta Flyers’ Podcast For ‘Star Trek: Deep Space Nine’ Rewatch

DS9 , Interview , Podcasts , TNG
Interview: Cirroc Lofton And Denise Crosby On Watching And Podcasting ‘Star Trek: TNG’ Season 1

DS9 , Shuttle Pod
The Shuttle Pod Celebrates The 30th Anniversary Of ‘Star Trek: Deep Space Nine’
As a kid, I used to tell my friends if there was a 24/7 Star Trek: Deep Space Nine channel, I would absolutely tune in regularly.
I think my kid self just called my bluff.
I watch Star Trek on Pluto TV nearly everyday.
Same here. It’s great to have on ‘in the background’ while making dinner, etc.
Someone can dedicate an entire channel to Deep Space Nine, but the owners of Deep Space Nine can’t convert it to HD? To quote a droid from another franchise, “This is madness!”
I feel you but like hear me out
I think one of these things is far more expensive than the other
There’s an old adage about gratitude that goes something to the effect of, “If you find yourself having to walk for miles because your car broke down, try to remember how many people would be thrilled to be able to take that walk.” In this case, if your vision is good enough to make a real distinction between standard-def and HD, be happy you can see that well. Many others can’t, myself included.
Damn, that really sucks, Michael. Is it cataract-related or something less treatable? I’m always worried about vision myself, given the connection between diabetes and blindness.
The Pluto news is pretty good, as I still haven’t gotten season 5 on dvd, which is where my favorite Eddington show appears, plus the baseball card episode.
My vision has been terrible for my entire life, with myopia so severe that it lands me in the top 95th percentile. The good news is that it’s been correctable, so while I was never going to fly the space shuttle (or anything else) I’ve been able to wear contacts that allow me to drive and do just about everything a person with normal vision can do. It’s gotten somewhat worse lately, where I’ll now be in the ridiculous position of having to wear glasses on top of my contacts to see things up close, but that’s my particular burden to bear and I’ll deal with it while still indulging my passion for photography. I’m 65, am in mostly good health, still have the greater percentage of my hair and can press 200+, for all of which I’m grateful. Don’t cry for me, Argentina. 😊
I actually got a doctor’s excuse to get out of swimming in high school owing to what he described as my 20/500 vision (I think it was a bit less than that), saying I’d keep colliding with the pool walls unless the school wanted to make me prescription scuba masks (oddly enough, I met up with my dad later that same year and found out he actually did have a prescription scuba mask for snorkeling, which I guess makes sense given he lived in Hawaii and was probably as nearsighted as I was.)
Wow, my long-lost nearsighted brother. (My actual brother’s eyesight is even worse than my own.) After losing an expensive pair of glasses — there’s no other kind with my prescription — I came to understand that swimming in the ocean would always be problematic for me.
In Canada Pluto has one Star Trek channel and the play TOS and TNG on it. They’ll play roughly 4 or 5 TOS episodes then same amount of TNG episodes and keep rotating.
Yeah, I am envious of those in the US because they not only have two channels plus the new DS9 channel, they also have TOS, TNG and DS9 on demand. To top it off, Pluto US also has a Stargate channel showing episodes of SG1 and Atlantis. Up here we have one live only channel of TOS and TNG.
That said, I guess I should not complain because CTV Sci-Fi channel does show multiple episodes of TOS, TNG, Voyager, DS9 and Enterprise along with SG1 throughout the week and weekend mornings.
Here in Australia our pluto.tv doesn’t have any Star Trek
Thanks for keeping things in perspective. Hopefully sometime soon, Pluto adds a Trek channel to their lineup down under.
Wow great news! I’m amazed how popular DS9 has become the last few decades.
Yup pretty cool. IMHO DS9 is perhaps my favorite series competing head to head with TNG followed by TOS. SNW is also up there too, but not quite in the league with the top 3!
I recently fully rewatched the series (first time since it aired and I was a wee child glued to it) and I can’t honestly say I know what I think of it. Season four and five were almost exclusively excellent and the rest was ups and downs, save for season seven which was almost complete garbage. I loved the best of it and couldn’t stand a lot of it. Do I love the show?? I don’t even know. It left a bad taste but when I look a bit further back I remember how much I loved some of it…and I’m glad it’s getting some love b/c I think the best of it is among the best of Trek. But I can’t say I want to go back to this style of making a season of television. 🫠 I’d love to see some of the available characters again, though, some kind of follow up that did away with the worst flaws. (I do need to read the comics.) (And in case anyone wants to do the toxic nostalgia song n dance, I only did that rewatch b/c modern Trek star Tawny Newsome talks DS9 up at every opportunity. And overall it left me so grateful for the modern era and newly appreciative of the work done in the past. And pleased that I’ll get more of the references in Lower Decks now I’m refreshed, lol)
I agree, for the most part. Season 7 is not garbage and had some of the best episodes, but the final 10 were rough. But I think that is what left that “bad taste.”
If were to rank the 7 seasons of DS9 from best to worst:
4 5 6 3 7 2 1
That is how I feel now, but that could change after another re-watch.
Nothing wrong with liking what you like. I happen to think that the best of DS9 was as good a Star Trek as we’re ever likely to get, but that doesn’t mean the series as a whole was anything like perfect.
I still haven’t seen several trill-oriented eps because the whole subject doesn’t speak to me. Doesn’t stop me from being blown away by a number of great and good eps, though.
am thinking by season, it would probably be, top to bottom: 4 6 3 5 7 2 1
Looks like the “More Star Trek” channel is now the Star Trek: Voyager channel. So DS9 and Voyager both have their own “new” channels.
I’m guessing the More Star Trek channel will add something to the rotation besides Voyager. Otherwise, it seems like they would have renamed it the Star Trek Voyager channel when they were making their changes. I would love for every ST series to have its own channel, or at least for TOS to have its own channel, but I guess we’ll see what happens.
I would imagine Pluto’s unpausable live streaming format probably doesn’t work the best for DS9’s serialized nature. But definitely can cater to the fan wanting o drop in and “trek channel surf”
Pluto needs the ability to pause live content . Even if it’s a max of 15 mins. You can do that with current cable.
Right now it’s literally like watching over the air TV before VCRs were created. No ability to pause or even ” record ” what your seeing.
I disagree. I actually like the lack of ability to pause to mimic the “before-time.”
Also, if you have Paramount + or the shows on disc, then you can pause them that way.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 December 2020
K 2P 2.1 (TREK-1) potassium channel activation protects against hyperoxia-induced lung injury
- Tatiana Zyrianova 1 ,
- Benjamin Lopez 1 ,
- Riccardo Olcese 2 , 3 ,
- John Belperio 4 ,
- Christopher M. Waters 5 ,
- Leanne Wong 1 ,
- Victoria Nguyen 1 ,
- Sriharsha Talapaneni 1 &
- Andreas Schwingshackl 1
Scientific Reports volume 10 , Article number: 22011 ( 2020 ) Cite this article
2616 Accesses
14 Citations
3 Altmetric
Metrics details
- Cell biology
- Mechanisms of disease
No targeted therapies exist to counteract Hyperoxia (HO)-induced Acute Lung Injury (HALI). We previously found that HO downregulates alveolar K 2P 2.1 (TREK-1) K + channels, which results in worsening lung injury. This decrease in TREK-1 levels leaves a subset of channels amendable to pharmacological intervention. Therefore, we hypothesized that TREK-1 activation protects against HALI. We treated HO-exposed mice and primary alveolar epithelial cells (AECs) with the novel TREK-1 activators ML335 and BL1249, and quantified physiological, histological, and biochemical lung injury markers. We determined the effects of these drugs on epithelial TREK-1 currents, plasma membrane potential (Em), and intracellular Ca 2+ (iCa) concentrations using fluorometric assays, and blocked voltage-gated Ca 2+ channels (Ca V ) as a downstream mechanism of cytokine secretion. Once-daily, intra-tracheal injections of HO-exposed mice with ML335 or BL1249 improved lung compliance, histological lung injury scores, broncho-alveolar lavage protein levels and cell counts, and IL-6 and IP-10 concentrations. TREK-1 activation also decreased IL-6, IP-10, and CCL-2 secretion from primary AECs. Mechanistically, ML335 and BL1249 induced TREK-1 currents in AECs, counteracted HO-induced cell depolarization, and lowered iCa 2+ concentrations. In addition, CCL-2 secretion was decreased after L-type Ca V inhibition. Therefore, Em stabilization with TREK-1 activators may represent a novel approach to counteract HALI.
Similar content being viewed by others
WKYMVm hexapeptide, a strong formyl peptide receptor 2 agonist, attenuates hyperoxia-induced lung injuries in newborn mice
Young Eun Kim, Won Soon Park, … Yun Sil Chang
NLRX1 knockdown attenuates pro-apoptotic signaling and cell death in pulmonary hyperoxic acute injury
Hye Rin Kim, Mi Na Kim, … Myung Hyun Sohn
Molsidomine decreases hyperoxia-induced lung injury in neonatal rats
Mehmet Aslan, Ismail Kursat Gokce, … Ramazan Ozdemir
Introduction
Oxygen supplementation (hyperoxia; HO) is the most frequently administered therapy in hospitalized patients and the mainstay of treatment for hypoxic respiratory failure, regardless of its etiology 1 . Clinically, supra-physiologic levels of oxygen tension are often tolerated and perceived as a safety net against hypoxemia 2 . As a result, in the US each year approximately 800,000 patients are exposed to HO therapy at a cost of $1.8 billion to the health care budget 3 . Importantly, the degree and duration of HO exposure positively correlate with patient morbidity and mortality rates 4 , 5 , 6 .
Although oxygen therapy can be a life-saving intervention, ample experimental and clinical evidence demonstrates that excessive levels of oxygen supplementation can also initiate and accelerate existing lung injury (HO-induced acute lung injury; HALI). Animal models of HALI have been particularly helpful in investigating the underlying mechanisms 7 , and studies in healthy adults showed that HO exposure causes tracheobronchitis and changes in vital capacity, diffusing capacity, and lung permeability within only six hours, and with a severity that is proportional to the degree of HO exposure 8 , 9 , 10 , 11 , 12 , 13 . Experimentally, a similar dose- and time-dependent inflammatory response to HO can be reproduced in animal models of HALI 14 , 15 , 16 , demonstrating close similarities in lung injury phenotypes between animals and humans 15 , 17 , 18 , 19 , 20 . From these studies we learned that the molecular mechanisms underlying HALI are complex and include extensive alterations in inflammatory cytokine secretion 14 , 21 , 22 . Both alveolar epithelial and endothelial cells are injured by HO, but the epithelial layer is the first line of defense against inhaled HO 23 , 24 , 25 .
Currently, minimizing the duration and amount of HO exposure of patients (“permissive hypoxemia”) represents the only clinical approach to limit HALI, and so far no molecular targets have been identified that translate into improved patient outcomes 26 . However, minimizing HO exposure is complicated by the lack of consensus in defining the lower limits of permissive hypoxemia, which would allow us to clinically differentiate beneficial from injurious levels of HO therapy 27 , 28 .
In our search for new molecular targets against HALI, we recently identified epithelial K 2P 2.1 (TREK-1) K + channels as important regulators in the development and progression of HALI 29 , 30 , 31 , 32 . TREK-1 channels belong to the family of 2-pore domain (K2P) K + channels, which are generally known for their unusual gating properties leading to so-called “leak K + currents” that stabilize the resting plasma membrane potential (Em) 33 , 34 . In general, K2P channels, including TREK-1, are widely expressed in body tissues 35 , 36 , 37 , 38 , 39 , 40 , 41 , but their role in the lung remains largely unknown. Using in vivo and in vitro models of HALI, we previously discovered that HO exposure decreases the expression of TREK-1 channels in mouse lungs and alveolar epithelial cells, and accelerates alveolar inflammation and barrier dysfunction 30 , 42 , 43 . These findings sparked the hypothesis that despite HO-mediated TREK-1 downregulation, pharmacological activation of the remaining subset of TREK-1 channels can protect against HALI. To test this hypothesis, in this study we explored the potential protective effects and underlying mechanisms of two novel TREK-1 activating compounds (ML335, BL1249) using in vivo and in vitro models of HALI.
Intra-tracheal administration of TREK-1 activating compounds protects mice against HO-induced acute lung injury (HALI)
Building on our previous findings that HO downregulates TREK-1 expression 42 , we determined whether pharmacological activation of the remainder subset of TREK-1 channels can counteract the injurious effects of HO on mouse lungs. We treated WT mice with once-daily intra-tracheal ( i.t. ) injections of the TREK-1-activating compounds ML335 or BL1249 44 , 45 , or an equimolar drug vehicle control, for a total of 3 injections over the 72-h HO or room air (RA) exposure period. Histological analysis (Fig. 1 A) and blinded Lung Injury Scoring (LIS; Fig. 1 B) of H&E-stained mouse lung sections revealed that under RA conditions administration of ML335 and BL1249 had no damaging effect on lung histology. As expected, exposure of mice to HO caused significant inflammatory changes (panel d), as also evidenced by an increase in LIS. Importantly, once-daily i.t. injections of ML335 or BL1249 during HO exposure substantially reduced these HO-induced injurious effects (Fig. 1 A panels e and f, and B). Similarly, analysis of physiological parameters of lung injury also revealed protective effects of TREK-1 activation in HO-exposed mice, as evidenced by improvements in quasi-static lung compliance (Fig. 1 C), and a reduction in BAL fluid protein levels and total cell counts (Fig. 1 D, E). These data suggest that pharmacological activation of TREK-1 channels can counteract HALI in an experimental mouse model.
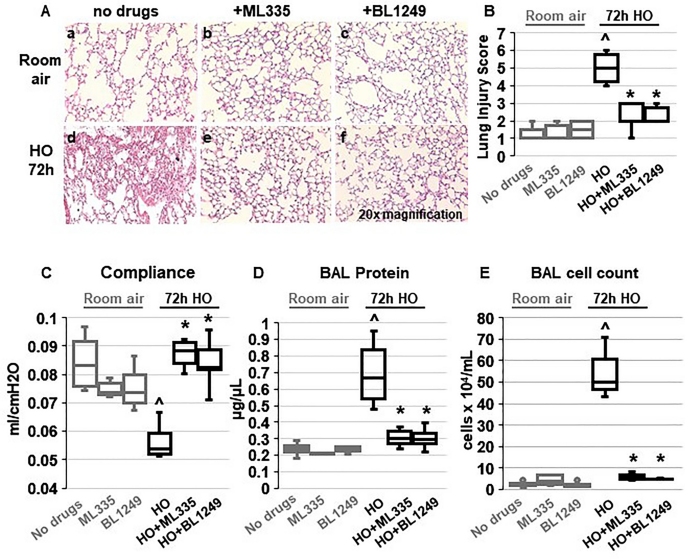
TREK-1 activation with the novel compounds ML335 and BL1249 protects form HO-induced lung injury: ( A ) Representative images of H&E-stained lung sections of WT mice exposed to either room air (panels a-c) or HO (panels d-f) for 72 h. All mice received once-daily, intra-tracheal ( i.t. ) injections of ML335, BL1249, or a vehicle control (no drugs) via brief endotracheal intubation. HO exposure caused significant lung injury (panel d), which was ameliorated by concomitant treatment with ML335 or BL1249 (panels e, f). ( B ) Summary of cumulative Lung Injury Scores of n = 5 independent experiments. ( C – E ) The HO-induced decrease in semi-static lung compliance, and increase in BAL fluid total protein and cell count were counteracted by ML335 and BL1249. Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; n = 5–9; ^compared to mice injected with a vehicle control and exposed to room air (no drugs), *compared to HO exposed mice; p ≤ 0.05.
TREK-1 activation decreases inflammatory cytokine concentrations in the BAL fluid of HO exposed mice
To investigate whether the TREK-1-mediated improvements in histological and physiological lung injury parameters are associated with a reduction in inflammatory cytokine concentrations in HO exposed mice, we measured IL-6, IP-10, CCL-2, TNF-α, MIP-1α and IL-10 concentrations in BAL fluid (Fig. 2 ). We found that under room air conditions once-daily i.t. injections of ML335 or BL1249 had no effect on baseline cytokine secretion. Exposure of mice to 72 h HO resulted in an increase in IL-6, IP-10, CCL-2, TNF-α and IL-10 concentrations in the BAL fluid. Importantly, once-daily i.t. injections with ML335 or BL1249 during the 72 h of HO exposure decreased HO-induced IL-6 and IP-10 levels in the BAL fluid, but not CCL-2, TNF-α or IL-10. MIP-1α concentrations were neither affected by HO exposure nor treatment of mice with the TREK-1 activating compounds.
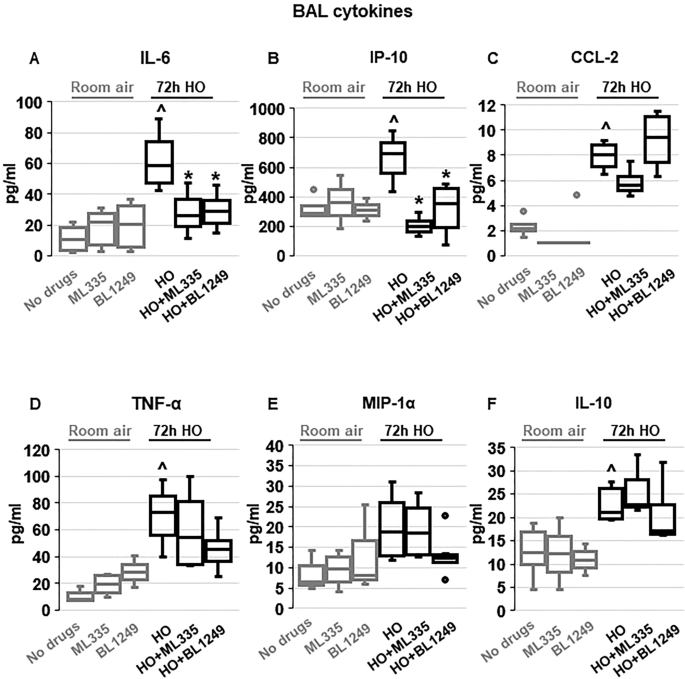
Effects of ML335 and BL1249 on BAL fluid cytokine concentrations ( A – F ): HO exposure increased IL-6, IP-10, CCL-2, TNF-α, and IL-10 concentrations, but not MIP-1α. Once-daily i.t . treatment with ML335 or BL1249 decreased HO-induced IL-6 and IP-10 levels, but not CCL-2, TNF-α or IL-10. Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; n = 5–9; ^compared to mice exposed to room air and treated with a vehicle control (no drugs), *compared to HO exposed mice; p ≤ 0.05.
TREK-1 activity regulates inflammatory cytokine secretion from primary mouse AT2 cells
To evaluate whether the protective effects of TREK-1 activation observed in vivo were due to TREK-1-mediated attenuation of inflammatory cytokine secretion from alveolar epithelial cells, we exposed freshly-isolated mouse AT2 cells to HO or RA in the presence or absence of ML335 or BL1249, and quantified inflammatory cytokine secretion in culture supernatants (Fig. 3 ). We chose the shorter (24-h) HO exposure period (compared to 72 h in vivo) for freshly isolated AT2 cells, since in this cell type 72 h of HO exposure resulted in > 60% AT2 cell death (data not shown). Importantly, real-time PCR experiments and immunofluorescence (IF) microscopy imaging confirmed HO-induced TREK-1 downregulation after 24 h in this cell-type (Supplementary Fig. 1 A,B). Similar to our findings in the BAL fluid, HO exposure increased secretion of IL-6 and CCL-2 from freshly isolated mouse AT2 cells, and this effect was counteracted by concomitant treatment of cells with the TREK-1 activators ML335 or BL1249. Furthermore, HO exposure did not induce MIP-1α secretion from AT2 cells, similar to our findings in the BAL fluid. In contrast to our findings in the BAL fluid, HO exposure did not induce IP-10, TNF-α or IL-10 secretion from primary AT2 cells, and treatment with ML335 or BL1249 had no effect on the secretion of these cytokines at baseline or after HO exposure (Fig. 3 ).
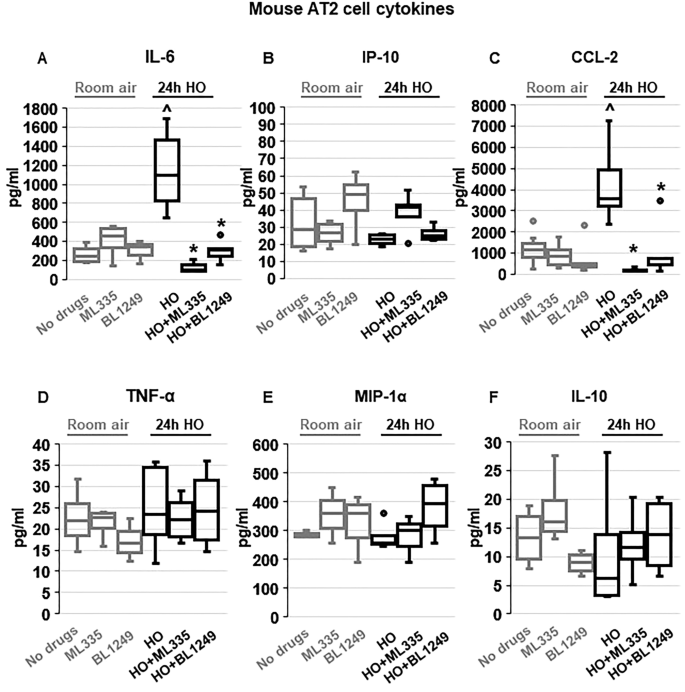
TREK-1 activation with ML335 and BL1249 regulates cytokine secretion from primary mouse AT2 cells: HO exposure increased IL-6 and CCL-2 secretion, which were inhibited by concomitant treatment with ML335 or BL1249 ( A , C ). In contrast, IP-10, TNF-α, MIP-1α and IL-10 levels were not affected by TREK-1 activation in room air- or HO-exposed AT2 cells ( B , D , E , F ). Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; n = 5–9; ^compared to cells treated with a vehicle control and exposed to room air (no drugs), *compared to HO exposed cells; p ≤ 0.05.
TREK-1 activity regulates inflammatory cytokine secretion from primary human alveolar epithelial cells (HAECs)
To determine whether the TREK-1-mediated protective effects observed in mice and mouse AT2 cells can be reproduced in primary human alveolar epithelial cells (HAEC), we exposed HAEC to 72 h HO in the presence or absence of the TREK-1 activators ML335 or BL1249 (Fig. 4 ). Initial dose–response experiments revealed that BL1249 and ML335 are not cytotoxic at the doses used in this study (Supplementary Fig. 2 ). In contrast to primary mouse AT2 cells, viability of HAECs after 72 h HO exposure remained > 70% (data not shown), and this exposure period closely mimicked our in vivo model. Similar to our findings in primary mouse AT2 cells, HO exposure increased secretion of IL-6 and CCL-2 from HAECs, but did not induce TNF-α or MIP-1α secretion. Of note, overall concentrations of TNF-α and MIP-1α were quite low in these cells. In contrast to primary mouse AT2 cells but similar to BAL fluid, HO also increased secretion of IP-10 and IL-10 from HAECs. Importantly, treatment of HAECs with the TREK-1 activators ML335 or BL1249 inhibited the HO-induced secretion of IL-6, IP-10, CCL-2, and IL-10.
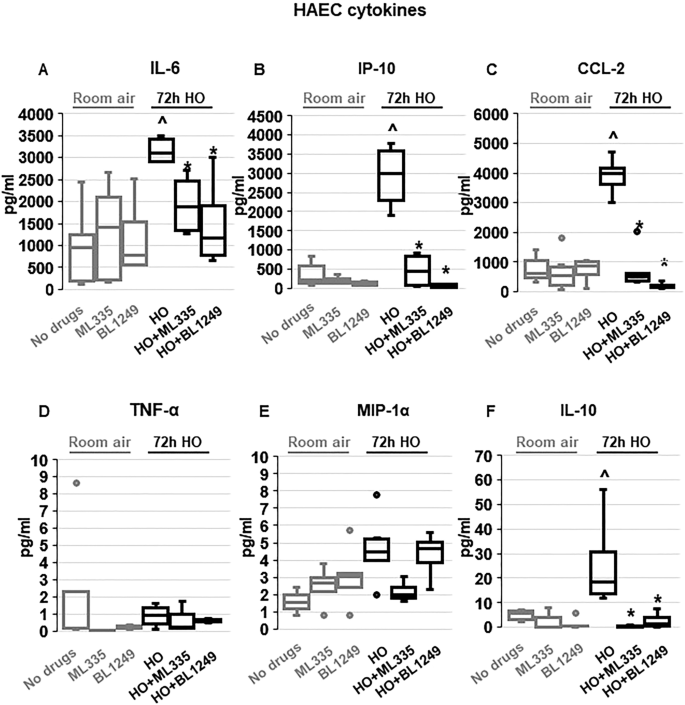
TREK-1 activation with ML335 and BL1249 regulates cytokine secretion from primary human alveolar epithelial cells (HAEC): HO exposure increased secretion of IL-6, IP-10, CCL-2 and IL-10, and this effect was counteracted by ML335 or BL1249 ( A , B , C , F ). In contrast, TNF-α and MIP-1α levels were not affected by TREK-1 activation in room air- or HO-exposed HAECs ( D , E ). Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; n = 4–8; ^compared to cells treated with a vehicle control and exposed to room air (no drugs), *compared to HO exposed cells; p ≤ 0.05.
Altogether, these findings suggest that TREK-1 activation regulates HO-induced inflammatory cytokine secretion both in vivo and in vitro, but differences can be observed between overall cytokine concentrations in BAL fluid and cultured primary epithelial cells.
ML335 and BL1249 activate TREK-1 currents in primary AT2 cells
Although the specificity of ML335 and BL1249 for TREK-1 channels has previously been validated in heterologous expression systems 44 , 46 , 47 , the effectiveness of these compounds has never been demonstrated in lung cells. To confirm that both compounds activate TREK-1 currents in a physiologically relevant system and cell type, we used fluorescence-based, K + -sensitive FLIPR assays to demonstrate the effects of ML335 and BL1249 on K + currents in primary mouse AT2 cells (Fig. 5 ). FLIPR assays exploit the permeability of thallium (Tl + ) for open K + channels 48 . After loading AT2 cells with the fluorescent dye, the addition of extracellular Tl + creates a concentration gradient for Tl + to enter the cells. The resultant increase in relative fluorescence is proportional to the open probability of plasma membrane K + channels, and thus represents a measure of the functional activity of K + channels. Therefore, under unstimulated conditions (no drugs), the Tl + -induced fluorescence represents the sum of background K + currents, while after ML335 or BL1249 treatment an increase in fluorescence represents activation of TREK-1-specific K + currents (Fig. 5 ). Our data show that under RA conditions both compounds, ML335 and BL1249, activate TREK-1-specific K + currents (Fig. 5 A). Importantly, these effects were maintained after 24 h of HO exposure (Fig. 5 B).
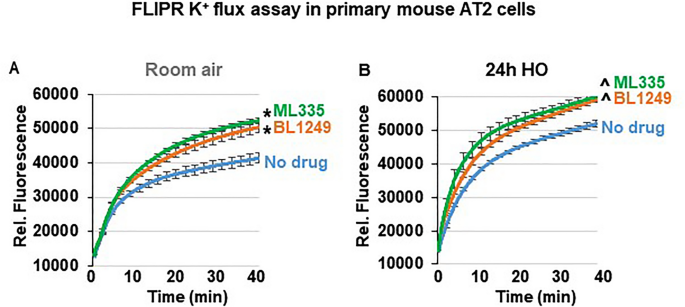
ML335 and BL1249 activate TREK-1 currents in primary mouse AT2 cells: Summary of n = 4–5 independent FLIPR curves (means ± SEM) showing that the TREK-1 activating compounds ML335 or BL1249 induce TREK-1 specific K + currents under both room air and HO conditions ( A , B ). In both room air and HO-treated cells, baseline background K + currents were observed (No drug). *compared to vehicle control (No drug) at room air, ^compared to vehicle control (No drug) after HO exposure; p ≤ 0.05, n = 4–5, individual experiments were run in triplicates.
Activation of TREK-1 channels hyperpolarizes the plasma membrane potential (Em) of primary AT2 cells
To determine whether the protective effects of ML335 and BL1249 are mediated by TREK-1-induced alterations in the Em, we performed Em-sensitive FLIPR assays on primary mouse AT2 cells under RA and HO conditions (Fig. 6 ). Once cells are loaded with the Em-sensitive fluorescent dye, a decrease in relative fluorescence represents Em hyperpolarization, whereas an increase in fluorescence represents Em depolarization. Since both TREK-1 activating compounds, ML335 and BL1249, had identical effects (i) in our in vivo model, (ii) on inflammatory cytokine secretion, and (iii) on TREK-1 current activation, we used only BL1249 for this part of the study. We found that under room air conditions, activation of TREK-1 channels with BL1249 results in Em hyperpolarization (= a decrease in fluorescence; Fig. 6 A) of primary AT2 cells. Similar to the observed activation of TREK-1 currents with BL1249 (Fig. 5 ), the BL1249-induced Em hyperpolarization also persisted after HO exposure (Fig. 6 B). Importantly, these studies also revealed that HO itself causes Em depolarization when compared to cells kept at RA, as evidenced by a higher baseline fluorescence value in HO-exposed cells (see RED arrows on the Y-axis → in Fig. 6 A, B and summarized in C).
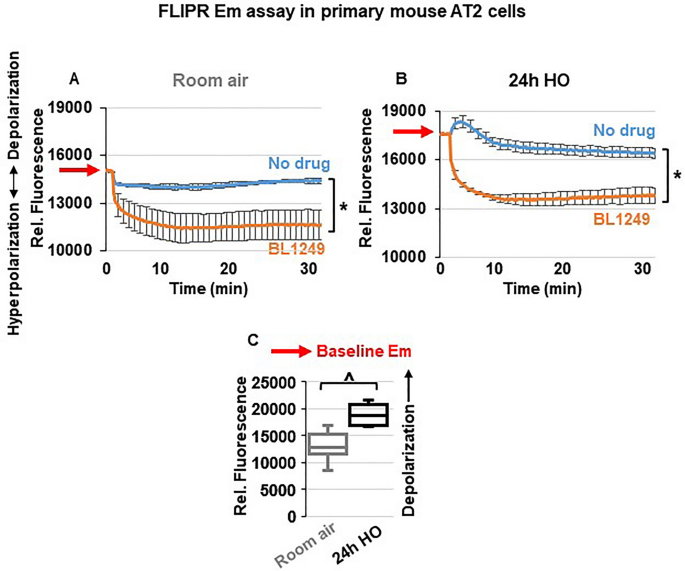
TREK-1 activation causes plasma membrane potential (Em) hyperpolarization: Representative curves of Em-sensitive FLIPR assays showing that TREK-1 activation with BL1249 causes Em hyperpolarization in primary mouse AT2 cells under both room air and HO conditions ( A , B ), as indicated by a decrease in fluorescence values. Red arrows on the Y-axis indicate relative fluorescence values reflective of the baseline Em value in room air and HO exposed AT2 cells, demonstrating that HO exposure itself causes Em depolarization (higher baseline fluorescence value in B than A; *BL1249 compared to no drug/vehicle control, p ≤ 0.05). ( C ) Summary of baseline Em values of RA- vs. HO-exposed AT2 cells averaging n = 6 independent experiments for each condition. Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; ^compared to room air exposed AT2 cells; p ≤ 0.05, individual experiments were run in triplicates.
TREK-1 activation decreases intracellular Ca 2+ (iCa) levels during HO exposure
Since inflammatory cytokine secretion is commonly associated with an increase in iCa concentrations, we used Fluo-4 assays to determine the effects of TREK-1 activation on iCa levels in primary mouse AT2 cells (Fig. 7 ). Importantly, following 24 h of HO exposure, AT2 cells contained higher iCa concentrations than cells kept at RA (PURPLE arrows on the Y-axis → in Fig. 7 A,B and summarized in C). In cells kept at RA, activation of TREK-1 channels and Em hyperpolarization with BL1249 had no effect on iCa levels, likely due to the already low iCa levels in resting cells (Fig. 7 A). In HO-exposed cells, on the other hand, TREK-1 activation with BL1249 decreased the HO-induced elevation in iCa levels (Fig. 7 B).
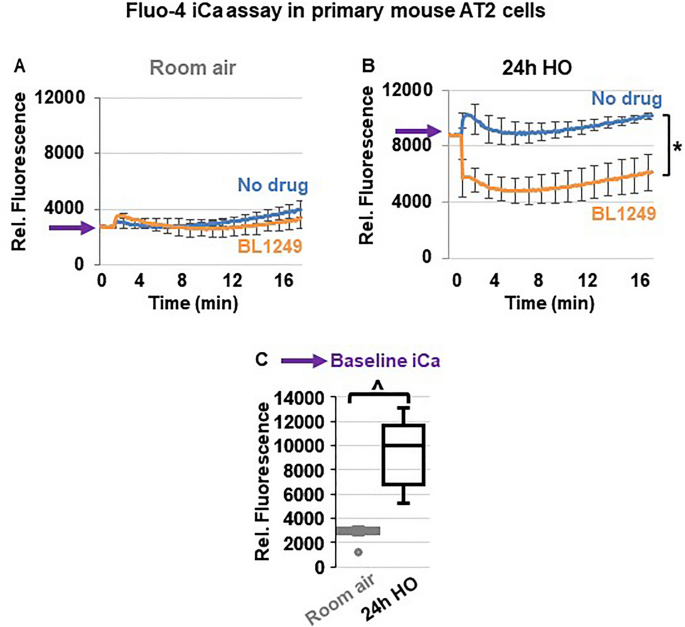
TREK-1 activation decreases intracellular Ca 2+ (iCa) concentrations in HO-exposed primary mouse AT2 cells: ( A , B ) Representative curves of Ca 2+ -sensitive Fluo-4 assays showing that HO-exposed AT2 cells contain higher iCa concentrations than RA-exposed cells, as indicated by an increase in fluorescence values (purple arrows on Y-axes). TREK-1 activation with BL1249 has no effect on iCa concentrations in RA-exposed cells, but decreases iCa levels in HO-exposed cells (*BL1249 compared to no drug/vehicle control, p ≤ 0.05). A summary of n = 6 independent experiments is shown in C ; data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; ^compared to room air exposed AT2 cells; p ≤ 0.05, individual experiments were run in triplicates.
Altogether, these findings highlight the TREK-1 activating effects and resultant Em hyperpolarization caused by ML335 and BL1249 in primary alveolar epithelial cells, and demonstrate that these effects persist under HO conditions, making TREK-1 activation a feasible approach to modulate the Em and iCa concentrations during HO exposure.
Regulation of inflammatory cytokine secretion by voltage-gated Ca 2+ (Ca V ) channels
Em depolarization, as observed with HO exposure and counteracted by TREK-1 activation, results in opening of Ca V channels in many cell types, and the resultant increase in iCa concentrations is commonly a trigger for downstream inflammatory cytokine secretion 49 , 50 . Therefore, we measured HO-induced cytokine secretion from primary mouse AT2 cells after blocking N- and P/Q-type Ca V channels with ω-conotoxin MVIIC 51 , and L-type Ca V channels with nifedipine 52 (Fig. 8 ). Since in primary AT2 cells HO exposure predominantly induced secretion of IL-6 and CCL-2 (Fig. 3 ), we focused on the role of Ca V channels in the secretion of these two cytokines. Interestingly, while HO-induced IL-6 secretion was not dependent on Ca V channel activity, CCL-2 secretion was inhibited by the L-type Ca V channel blocker nifedipine, but not by the N- and P/Q-type Ca V channel blocker ω-conotoxin MVIIC. Secretion of IP-10, TNF-α, MIP-1α and IL-10 from AT2 cells was not affected by ω-conotoxin MVIIC or nifedipine (data not shown).
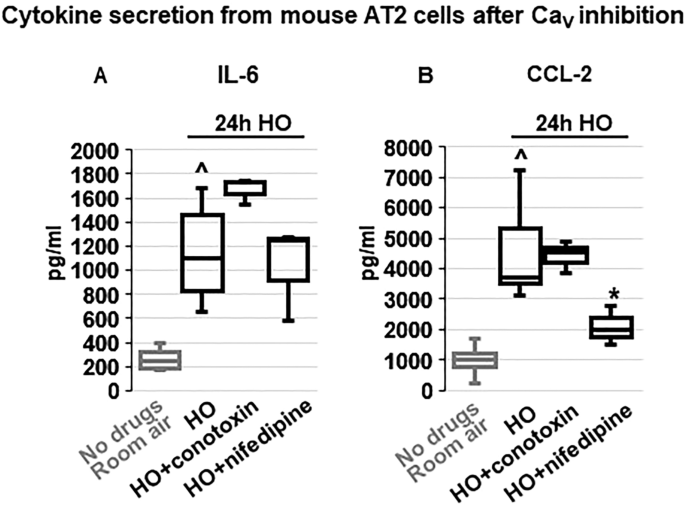
Effects of voltage-gated Ca 2+ channel (Ca V ) inhibition on cytokine secretion from primary mouse AT2 cells: HO exposure increased IL-6 ( A ) and CCL-2 ( B ) secretion compared to RA-treated cells. Inhibition of N- and P/Q-type Ca V channels with ω-conotoxin MVIIC or L-type Ca V channels with nifedipine revealed that IL-6 secretion occurred independently of Ca V channel activity, whereas CCL-2 secretion was dependent on L-type Ca V channels (inhibited by nifedipine) but not N- and P/Q-type channels (lack of ω-conotoxin MVIIC effect). Data are represented as Box-Whisker plots with medians, 1st and 3rd quartiles, and max and min values; n = 3–6; ^compared to cells treated with a vehicle control and exposed to room air (No drugs), *compared to HO exposed cells; p ≤ 0.05.
Altogether, these findings suggest that regulation of inflammatory cytokine secretion via TREK-1-induced Em hyperpolarization and inhibition of Ca V channel activation could explain some, but not all, of the TREK-1 protective effects seen in our in vivo model.
In this study we propose activation of TREK-1 K + channels as a potentially new therapeutic approach against HALI, since currently no targeted interventions exist that translate into improved patient outcomes. Recent in vitro studies suggest that overexpression of certain microRNAs (miR16, miR21-5) may protect cultured AT2 cells against HO-induced apoptosis 53 , 54 , 55 , and multiple biomarker studies have aimed at predicting the risk of HALI in patients 56 , 57 . In addition, neutralizing therapies against individual cytokines, including IL-6, TNF-α and CCL-2, have yielded variable results at best in improving inflammatory responses 58 , 59 , 60 , 61 .
Given these challenges, we are particularly interested in identifying strategies that can regulate multiple inflammatory pathways simultaneously, such as the manipulation of the plasma membrane potential (Em). We previously discovered that HO downregulates TREK-1 K + channel expression in lung tissue and alveolar epithelial cells, which correlates with worsening lung injury and alterations in multiple inflammatory cytokines (IL-6, CCL-2, RANTES, and IL-1β) 29 , 30 , 31 , 32 , 42 . Importantly, this HO-induced decrease in TREK-1 expression leaves a remainder subset of TREK-1 channels suitable for pharmacological activation. Although channels of the K2P family are known for their so-called “leak K + currents” (a constant, slow K + efflux that stabilizes the Em), TREK-1 channels are actually thought to be closed at baseline 34 , 62 . This idea is supported by our own data in alveolar epithelial cells (Fig. 5 ) showing that TREK-1 currents can readily be induced by our channel activators ML335 and BL1249 44 , 46 , 47 , thus making TREK-1 channels a feasible target for therapeutic activation.
So far, most of the biophysical characterization of TREK-1 channels has occurred under non-physiological conditions in heterologous expression systems 44 , 63 , 64 , and little is known about their functions in physiologically-relevant models. Our study is the first to (a) report the safety and efficacy of the novel TREK-1 activating compounds ML335 and BL1249 in an in vivo system, and (b) highlight the protective effects of TREK-1 activation in a lung injury model by measuring clinically relevant parameters. The only other reports suggesting a potentially protective role for TREK-1 activation used models of hypoxic-ischemic brain injury and atrial fibrillation/heart failure 65 , 66 , 67 . Interestingly, effects of single nucleotide polymorphisms (SNPs) in the human TREK-1 gene have been reported in the same two organs, and predict resistance to antidepressant medication 68 , and an increased risk for atrial tachycardias 69 . However, until now a similar protective effect for TREK-1 channels has not been reported in any other organ.
The importance of inflammatory mediators in the development and progression of HALI is well-established 14 , 70 , including the cytokines reported in this study: IL-6, IP-10, CCL-2, TNF-α, MIP-1α, and IL-10 17 , 21 . In general, IL-6, IP-10, TNF-α and MIP-1α are known for their proinflammatory properties, while CCL-2 can exert pro- 71 , 72 or anti-inflammatory 73 , 74 effects, and IL-10 is considered a predominantly anti-inflammatory cytokine 75 , 76 . More recently it has become increasingly clear that the inflammatory phenotypes observed in various lung injury models are determined by complex interactions between multiple cytokines. For example, despite the well-documented proinflammatory effects of IL-6 and its association with poor outcomes in ARDS patients 77 , IL-6 also induces anti-inflammatory IL-10 secretion as a counter-regulatory response 78 , and a recent study suggests that IL-6 protects mice from LPS- and mechanical ventilation-induced lung injury 79 . In our HALI model we found increased levels of both IL-6 and IL-10 in the BAL fluid of HO-exposed mice. Interestingly, while TREK-1 activation decreased HO-induced BAL fluid IL-6 levels, IL-10 levels remained elevated even after TREK-1 activation, potentially acting synergistically with the protective effects of TREK-1 activation. It is important to note that both lung resident and immune cells contribute to the cytokine levels measured in BAL fluid, and it is quite likely that in our in vivo model the TREK-1 activators affect cytokine secretion from multiple cell types. In this study we focused on epithelial cells since previously we did not find alterations in TNF-α release from TREK-1-deficient alveolar macrophages, and the single cell RNA-seq database LungGENS only reports low levels of TREK-1 postnatally in endothelial cells 42 , 80 .
Our results report for the first time (1) the expression of functional TREK-1 channels on primary mouse AT2 cells and human alveolar epithelial cells (HAEC), and (2) the effects of Em manipulation via TREK-1 channels on inflammatory cytokine secretion and iCa concentrations in a clinically relevant model of HALI. Since in clinical practice the timing of HO therapy is entirely under the control of the healthcare provider, administration of TREK-1 activators simultaneously with initiation of HO therapy is a clinically feasible approach. Of note, although in animal models the injurious effects of HO on previously healthy lungs have been extensively studied, in humans the exact degree and duration of HO exposure that results in symptomatic and clinically-relevant injury remains a matter of intense discussion 81 .
From studies in macrophages, neutrophils and mast cells, we learned that changes in the Em commonly precede secretory events 82 , 83 , but the molecular mechanisms regulating inflammatory cytokine secretion from lung resident cells remain incompletely understood. Furthermore, studies in lung endothelial cells, revealed that the resting Em can vary among cell phenotypes. Reported Em values in endothelial cells range from − 30 to − 60 mV 84 , 85 , and exposure of pulmonary artery endothelial cells to low oxygen concentrations (hypoxia) has been reported to cause Em depolarization 86 . Similar variations in Em depending on the cellular phenotype have also been documented in lung epithelial cells, including rat AT2 cells (− 30 mV) 87 , 88 , rabbit AT2 cells (− 60 mV) 89 , human bronchial epithelial cells (− 20 to − 45 mV) 90 , 91 , and nasal epithelial cells (− 15 to − 30 mV) 91 , 92 . Limited information form human ex vivo studies point towards Em values between − 15 and − 20 mV in bronchial epithelial cells 91 , 93 . Notably, other studies estimate the resting Em in AT2 cells as low as 0 to − 5 mV 94 , 95 . Despite these ranges in Em for lung resident cells, it is important to realize that the Em of epithelial and endothelial cells is much lower than the Em of excitable cells such as neurons and cardiomyocytes, in which the Em ranges between − 60 and − 90 mV 96 , 97 . Since these latter cell types are more hyperpolarized at baseline (i.e. more negative Em values), they require a much stronger depolarization stimulus for a biological response to occur, such as the opening of voltage-gated Ca 2+ (Ca V ) channels and subsequent Ca 2+ influx. In contrast, in the more depolarized epithelial and endothelial cells, a much smaller Em perturbation can reach the threshold for Ca V channel activation, and trigger downstream responses. Conversely, K + efflux, as caused by TREK-1 activation with BL1249 (Fig. 6 A), moves the Em away from this critical threshold towards more negative (hyperpolarized) Em values, and can counteract depolarization-induced cell activation processes.
HO-mediated depolarization events have been reported in mitochondrial membranes of pulmonary endothelial cells 98 , but our study is the first to show HO-induced Em depolarization in primary epithelial cells. Interestingly, in carotid body cells hypoxia, not hyperoxia, causes Em depolarization and increases iCa 2+ concentrations, while HO inhibits both of these processes 99 , 100 . In contrast to these studies, we demonstrate that primary epithelial cells respond to HO exposure by increasing iCa 2+ levels (Fig. 7 ), and we propose that this response is mediated by HO-induced Em depolarization that can be counteracted by TREK-1 activation (Figs. 6 , 7 ).
Interestingly, although it is well-known that both extracellular Ca 2+ influx and Ca 2+ release from intracellular stores can increase iCa 2+ levels, we found that in primary mouse AT2 cells only secretion of CCL-2, but not IL-6, IP-10, TNF-α, or MIP-1α, was dependent on Ca 2+ influx via Ca V channels (Fig. 8 ). The lack of effect of ω-conotoxin MVIIC on cytokine secretion suggests that Ca 2+ influx via N-, and P/Q-type Ca V channels is unlikely to contribute to these processes. In addition to the novelty and importance of our data, these findings also indicate that Ca 2+ release from intracellular stores is likely to be involved in the observed secretory processes.
Although upregulation of CCL-2 in bronchial and alveolar epithelial cells under inflammatory conditions is well-documented 101 , 102 , 103 , it remains a matter of intense discussion whether CCL-2 secretion in the lung is a Ca 2+ -dependent process, and may ultimately depend on the specific cell type and inflammatory environment. In both immortalized and primary lung epithelial cells, inhibition of Ca 2+ sensing, Ca 2+ influx, and iCa 2+ release all prevent CCL-2 secretion, and in some instances also IL-6 release 104 , 105 . Conversely, it is known that in immune cells CCL-2 itself can increase iCa 2+ concentrations 106 , demonstrating the complex interactions underlying CCL-2 secretion. One study showed that the chemotactic function of CCL-2 can occur in the absence of any changes in iCa 107 , and in an LPS-induced lung injury model inhibition of cellular Ca 2+ sensing receptors (CaSR) decreased IL-6 and TNF-α, but not CCL-2, concentrations in the serum and BAL fluid 104 .
Since in our model inhibition of Ca V channels decreased CCL-2 secretion but no other measured cytokines, we should consider the possibility that TREK-1-induced changes in Em could be directly sensed by a voltage-sensitive protein at the plasma membrane level. For this to occur, such a protein would need to contain one or more transmembrane segments with free charges that can induce a so-called “gating current” following an alteration in Em. Although membrane-bound voltage sensors are well-characterized in the brain and heart 108 , 109 , in the lung this important topic has yet to be explored.
We previously reported an important role for TREK-1 in HALI using a TREK-1-deficient mouse model 42 , which revealed a similar injurious phenotype as can be obtained with HO-induced TREK-1 downregulation 42 . In this study, we now shed some light on how TREK-1 may regulate downstream signaling cascades during HO exposure. Based on the current and our previous studies, we propose that the primary mechanism underlying the HO-mediated effects on TREK-1 signaling consists in a decrease in TREK gene and protein expression levels, rather than potential HO-mediated post-translational modifications of the TREK-1 protein structure. Of note, in HEK293 cells, posttranslational TREK-1 phosphorylation has been reported, and resulted in TREK-1 inhibition 110 . However, even if such changes occurred in the lung, they do not seem to interfere with the activation effects of BL1249 and ML335 on TREK-1 channels. Since BL1249 and ML335 are designed to bind and functionally activate wildtype TREK-1 channels, substantial HO-induced structural/posttranslational changes to the TREK-1 structure are unlikely the cause for our reported outcomes. In fact, one of the key findings of this study is that BL1249 and ML335 can activate TREK-1 channels and ameliorate injury despite any HO-induced changes in the intra- and extracellular cellular environments. Notably, we previously reported TREK-1 expression in both AT1 and AT2 cells from mouse lung slices, as well as mouse alveolar macrophages (AMs), but saw only weak TREK-1 staining in the mouse lung endothelium. Interestingly, in that study we also found that LPS-induced TNF-α release from mouse AMs appears to occur independently of TREK-1 31 , suggesting that epithelial TREK-1 channels are the primary target for BL1249 and ML335 in our HALI model.
In conclusion, we report for the first time the functional expression of TREK- 1K + channels on primary alveolar epithelial cells. We show that pharmacological activation of TREK-1 channels during HO exposure is a novel and clinically feasible approach to protect against HALI by reducing inflammatory cell recruitment and barrier dysfunction in the lungs, which may at least in part be mediated by inhibition of inflammatory cytokine secretion. However, additional studies are required to identify other potential effector mechanisms contributing to TREK-1-mediated protection, which should include ROS production, cell death pathways, and inflammasome activation.
Materials and methods
C57bl/6 wild-type (WT) mice aged 9–12 weeks were obtained from Jackson Laboratories ( www.jax.org ). Mice were housed in same-sex groups of up to 5 mice per cage and provided with food and water ad libitum. For experimental purposes, mice were age- and gender-matched as closely as possible.
Mouse hyperoxia (HO) exposure
Using a rodent HO chamber and a 5-L oxygen concentrator (DeVilbiss Healthcare, #525DS), we exposed mice to HO (F i O 2 = 0.8–0.9 inside the chamber) for 72 h in their native cages with free access to food and water. Temperature, humidity and oxygen concentrations were monitored continuously using commercially available sensors (AcuRite 00325A1 for temperature and humidity; Hudson-RCI5800 for oxygen concentrations). During HO exposure, mice lost less than 10% of weight and appeared overall healthy. No deaths were observed. Control mice were exposed to room air (RA) for the same time period in their native cages.
TREK-1 activating compounds
We used two novel TREK-1 activating compounds, ML335 and BL1249. ML335 has been synthesized and validated by our collaborator Dr. Minor at UCSF 44 , who provided this compound to us as gift. BL1249 has most recently become commercially available (Tocris) 45 . Stock solutions for ML335 (100 mM) and BL1249 (100 mM) were prepared in DMSO. For in vivo experiments, we used a final concentration of 100 μM ML335 and 200 μM BL1249 in sterile PBS. For in vitro experiments in primary cells, we used a final concentration of 100 μM (60 μg/kg) ML335 and 10 μM (100 μg/kg) BL1249 suspended in culture media. Vehicle controls for all experiments contained equimolar amounts of the DMSO.
Intra-tracheal injections
During the 72 h of RA or HO exposure, mice were injected once-daily intratracheally ( i.t. ) via brief endotracheal intubation with either 40μL of the TREK-1 activating compounds ML335, BL1249, or a vehicle control in sterile PBS. Briefly, for i.t. injections, mice underwent brief inhaled isofluorane (2–5%) anesthesia until they lost consciousness, and were then suspended by their incisors on a 3.0 silk suture mounted on a 45 degree-angled stand. The tongue was gently extracted from the mouth and moved to the side using blunt forceps in order to visualize the vocal cords. Using fiberoptic guidance, a 20-gauge angiocatheter was passed through the vocal cords into the subglottic area, and 40 μL of drug or vehicle control were injected with a micropipettor. Mice were then placed back into their native cages and allowed to recover under a warming lamp until fully awake. No perianesthetic deaths were associated with this procedure.
Quasi-static lung compliance measurements
Following RA or HO exposure, a tracheostomy was performed using an 18-gauge steel catheter under general ketamine/xylazine anesthesia (intraperitoneal, 10 mg/kg ketamine; 20 mg/kg xylazine). Quasi-static lung compliance was measured using the Flexivent system (SQIREC). Pressure–volume curves (P–V) were recorded, and each set of P–V curves was preceded by an inflation maneuver to total lung capacity to insure equal standard lung volumes for each experiment. Quasi-static lung compliance was calculated by fitting data derived from the P–V curves to the Salazar-Knowles equation as previously described 111 . Rectal temperatures were maintained in physiologic range using a heat lamp. All experiments were terminal.
Broncho-alveolar lavage (BAL) fluid collection and lung histology
Following Flexivent measurements, BAL fluid was collected from all mice using a 1 ml syringe attached to the tracheostomy catheter. Two wash-outs were performed with 1 ml PBS/0.6 mM EDTA for BAL protein and cell count determination, and 1 ml PBS/0.5% BSA for cytokine assays. All samples were immediately placed on ice. Total BAL protein concentrations were measured using the Bradford assay (BioRad), and total BAL cell counts were performed using a Diff-Quick stain (Fisher Scientific). Thereafter, lung tissue was harvested and processed for histological examination. Briefly, the lungs were gently retrograde perfused via the right ventricle with 10 ml ice-cold PBS to remove red blood cells. Lung tissue was then removed en bloc and immediately perfused and fixed in 4% formalin. Paraffin-embedded sections were cut into 4 µm thick tissues slices using a Microtome, and H&E-stained for histological analysis. Lung Injury Scores (LIS) were determined by an investigator blinded to the experimental conditions on H&E-stained lung sections as previously described, using the following 3 criteria: (1) interstitial and alveolar edema, (2) cellular infiltrate, and 3) parenchymal and perivascular hemorrhage. Each criterion was assigned a score between 0–3, with “0” representing no injury, “1” representing mild injury, “2” representing moderate injury, and “3” representing severe injury. Five randomly assigned high power fields per slide were scored under 40 × magnification on a Motic AE20/21 inverted microscope, and scores were averaged for each criterion. Using the sum of these averages, a composite histological LIS was calculated for each experimental group.
Primary mouse and human alveolar epithelial cells
Primary mouse alveolar type-2 cells (AT2) cells were freshly isolated as previously described 112 . We obtained in average 3–5 × 10 6 AT2 cells per mouse lung with > 90% purity as assessed by immunostaining for pro-SPC. All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of California Los Angeles. Freshly isolated AT2 cells were seeded to 70–80% confluence at a density 3.5 × 10 6 cells per well in 6-well tissue culture plates coated with fibronectin. Cells were maintained in DMEM cell culture medium containing 10% FBS, 4 mM glutamine, 1% penicillin/streptomycin, and 0.25 µM amphotericin B. All experimental interventions were started on day 2 after AT2 cell isolation.
Primary Human Alveolar Epithelial Cells (HAEC) were purchased from ScienCell (#3200), cultured according to the company’s instructions, and used at a passage numbers < P5. Since these cell suspensions are directly isolated from donated human lung tissue, they contain mixed populations of AT1 and AT2 cells.
HO exposure of cells
HO exposure of cells was performed using a cell culture-compatible HO chamber. HAEC were exposed to 72 h of HO to mimic our in vivo HO protocol. Since in freshly isolated mouse AT2 cells we observed substantial cell death after 72 h of HO exposure (F i O 2 0.8–0.9), we limited HO exposure to 24 h for these cells. Controls for each cell type were cultured at room air for the respective time intervals. During the HO or RA exposure period, cell suspensions were treated with a one-time dose of the TREK-1 activating compounds ML335 (100 μM) or BL1249 (10 μM), or an equimolar DMSO vehicle control. Under all experimental conditions cell viability remained greater than 75% as determined by Trypan Blue staining. To assure that BL1249 and ML335 were not cytotoxic at the doses used, we performed dose–response experiments using two cell viability assays, CCK-8 (APExBIO) and XTT (Biotium).
TREK-1 gene and protein expression
We used real-time PCR and IF microscopy to confirm HO-induced TREK-1 downregulation after 24 h in freshly isolated mouse AT2 cells. Briefly, for PCR experiments total RNA was isolated using a Qiagen RNeasy Mini Kit (Hilden, Germany), 1 μg RNA was reverse transcribed with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and amplified by semi-quantitative real-time PCR (TaqMan) with primers specific for TREK-1 (KCNK2; Applied Biosystems). For IF microscopy, mouse AT2 cells were fixed with 4% paraformaldehyde and then incubated with an anti-TREK-1 primary antibody (Alomone, 1:200) at 4 °C overnight, followed by probing with a species-specific secondary antibody (1:1000; Abcam) for one hour at room temperature. Nuclei were counterstained with Fluoro Gel II mounting medium containing DAPI (EMS). All images were recorded using Zen 2009 Light Edition software version 5.5 (Zeiss; https://www.zeiss.com/microscopy/us/products/microscope-software/zen-lite.html ).
Cytokine measurements by ELISA
Cytokine concentrations were quantified in BAL fluid and cell culture supernatants after centrifugation at 8000 rpm for 5 min. Briefly, 100 μL of sample was loaded into 96-well ELISA plate, and analyzed following the manufacturer’s instructions. All samples were run in triplicates and values are displayed in pg/mL. Species-specific ELISA kits were purchased from the following vendors: IL-6 (BD Biosciences), IP-10 (mouse: R&D Systems; human: BD Biosciences), CCL-2 (BD Biosciences), TNF-α (BD Biosciences), MIP-1α (R&D Systems), IL-10 (R&D Systems).
FLIPR and Fluo-4 assays for K + flux, plasma membrane potential (Em), and intracellular Ca 2+ (iCa) measurements
K + channel activity and Em measurements were performed using commercially available FLIPR assays (Molecular Devices, #R8222 and #R8126, respectively), and Fluo-4 assays (Invitrogen, #F36206) for iCa measurements. All three assays were performed following the manufacturer’s instructions. Briefly, for all assays 30,000 cells/well were seeded into dark-walled, clear-bottom 96-well plates (Grenier Bio-One, #655090), and cultured in growth medium overnight. The next day, cells were washed once and incubated at 37 °C with the respective loading dye for 60 min for K + channel activity assays, and 30 min for Em and Fluo-4 assays. In all assays, fluorescence traces were recorded for 1 min to reach a stable baseline before the addition of any drugs. All plates were analyzed using a BioTek Synergy-2 fluorescence plate reader. Data points were collected and integrated every 7 s. To determine whether an increase in iCa concentrations was due to Ca 2+ influx via voltage-gated Ca 2+ (Ca V ) channels, we blocked N- and P/Q-type Ca V channels with ω-conotoxin MVIIC (1 μM), and L-type Ca V channels with nifedipine (10 μM).
Statistical analysis
Quasi-static lung compliance, BAL protein and cell counts, LIS values, cytokine concentrations, and FLIPR and Fluo-4 data are represented as Box-Whisker plots with median values, 1st and 3rd quartiles, and maximum and minimum values. FLIPR curves in Figs. 5 A,B, 6 A,B, and 7 A,B show mean + SEM values. Data were analyzed using the unpaired student t-test, multivariate analysis of variance (ANOVA), and pairwise comparison of means using the Tukey–Kramer method to adjust for multiple comparisons. All statistical analyses were performed using GraphPad Prism 7 software (version 6.04, La Jolla, CA; https://www.graphpad.com/ ), and p values p ≤ 0.05 were considered significant.
Study approval
Approval for all experiments was obtained from the “University of California Los Angeles Animal Research Committee (ARC). All experiments were performed in accordance with our institutional protocols, guidelines and recommendations.
Scala, R. & Heunks, L. Highlights in acute respiratory failure. Eur. Respir. Rev. https://doi.org/10.1183/16000617.0008-2018 (2018).
Article PubMed Google Scholar
Suzuki, S., Eastwood, G. M., Peck, L., Glassford, N. J. & Bellomo, R. Current oxygen management in mechanically ventilated patients: A prospective observational cohort study. J. Crit. Care 28 , 647–654. https://doi.org/10.1016/j.jcrc.2013.03.010 (2013).
Kim, V., Benditt, J. O., Wise, R. A. & Sharafkhaneh, A. Oxygen therapy in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 5 , 513–518. https://doi.org/10.1513/pats.200708-124ET (2008).
Article PubMed PubMed Central Google Scholar
Girardis, M. et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The oxygen-ICU randomized clinical trial. JAMA https://doi.org/10.1001/jama.2016.11993 (2016).
Hale, K. E., Gavin, C. & O’Driscoll, B. R. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg. Med. J. 25 , 773–776. https://doi.org/10.1136/emj.2008.059287 (2008).
Article CAS PubMed Google Scholar
O’Driscoll, B. R., Howard, L. S., Bucknall, C., Welham, S. A. & Davison, A. G. British Thoracic Society emergency oxygen audits. Thorax 66 , 734–735. https://doi.org/10.1136/thoraxjnl-2011-200078 (2011).
Dias-Freitas, F., Metelo-Coimbra, C. & Roncon-Albuquerque, R. Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir. Med. 119 , 23–28. https://doi.org/10.1016/j.rmed.2016.08.010 (2016).
Beckett, W. S. & Wong, N. D. Effect of normobaric hyperoxia on airways of normal subjects. J. Appl. Physiol. 1985 (64), 1683–1687. https://doi.org/10.1152/jappl.1988.64.4.1683 (1988).
Article Google Scholar
Caldwell, P. R., Lee, W. L. Jr., Schildkraut, H. S. & Archibald, E. R. Changes in lung volume, diffusing capacity, and blood gases in men breathing oxygen. J. Appl. Physiol. 21 , 1477–1483. https://doi.org/10.1152/jappl.1966.21.5.1477 (1966).
Davis, W. B., Rennard, S. I., Bitterman, P. B. & Crystal, R. G. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N. Engl. J. Med. 309 , 878–883. https://doi.org/10.1056/nejm198310133091502 (1983).
Davis, W. B. et al. Pulmonary oxygen toxicity. Bronchoalveolar lavage demonstration of early parameters of alveolitis. Chest 83 , 35s (1983).
Article CAS Google Scholar
Dolezal, V. The effect of longlasting oxygen inhalation upon respiratory parameters in man. Physiol. Bohemoslov. 11 , 149–158 (1962).
CAS PubMed Google Scholar
Sackner, M. A., Landa, J., Hirsch, J. & Zapata, A. Pulmonary effects of oxygen breathing. A 6-hour study in normal men. Ann. Intern. Med. 82 , 40–43 (1975).
Helmerhorst, H. J. F. et al. Hyperoxia provokes a time- and dose-dependent inflammatory response in mechanically ventilated mice, irrespective of tidal volumes. Intensive Care Med. Exp. 5 , 27. https://doi.org/10.1186/s40635-017-0142-5 (2017).
de los Santos, R. et al. One hundred percent oxygen lung injury in adult baboons. Am. Rev. Respir. Dis. 136 , 657–661. https://doi.org/10.1164/ajrccm/136.3.657 (1987).
McElroy, M. C. et al. Nitric oxide attenuates lung endothelial injury caused by sublethal hyperoxia in rats. Am. J. Physiol. 272 , L631-638. https://doi.org/10.1152/ajplung.1997.272.4.L631 (1997).
Bhandari, V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front. Biosci. 13 , 6653–6661 (2008).
Kallet, R. H. & Matthay, M. A. Hyperoxic acute lung injury. Respir. Care 58 , 123–141. https://doi.org/10.4187/respcare.01963 (2013).
Lv, R. et al. Advances in the therapy of hyperoxia-induced lung injury: Findings from animal models. Undersea Hyperb. Med. 41 , 183–202 (2014).
PubMed Google Scholar
Crapo, J. D. Morphologic changes in pulmonary oxygen toxicity. Annu. Rev. Physiol. 48 , 721–731. https://doi.org/10.1146/annurev.ph.48.030186.003445 (1986).
Bhandari, V. & Elias, J. A. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic. Biol. Med. 41 , 4–18. https://doi.org/10.1016/j.freeradbiomed.2006.01.027 (2006).
Lee, P. J. & Choi, A. M. Pathways of cell signaling in hyperoxia. Free Radic. Biol. Med. 35 , 341–350 (2003).
Kondrikov, D., Gross, C., Black, S. M. & Su, Y. Novel peptide for attenuation of hyperoxia-induced disruption of lung endothelial barrier and pulmonary edema via modulating peroxynitrite formation. J. Biol. Chem. 289 , 33355–33363. https://doi.org/10.1074/jbc.M114.585356 (2014).
Article CAS PubMed PubMed Central Google Scholar
Pagano, A. & Barazzone-Argiroffo, C. Alveolar cell death in hyperoxia-induced lung injury. Ann. N. Y. Acad. Sci. 1010 , 405–416 (2003).
Article ADS CAS Google Scholar
Roper, J. M. et al. In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 286 , L1045-1054. https://doi.org/10.1152/ajplung.00376.2003 (2004).
Article ADS CAS PubMed Google Scholar
Abdelsalam, M. & Cheifetz, I. M. Goal-directed therapy for severely hypoxic patients with acute respiratory distress syndrome: Permissive hypoxemia. Respir. Care 55 , 1483–1490 (2010).
Tasker, R. C. Hyperoxemia and death of critically ill: Is there a problem of confounding by indication or outcome?. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.201909-1860LE (2019).
Barrot, L. et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N. Engl. J. Med. 382 , 999–1008. https://doi.org/10.1056/NEJMoa1916431 (2020).
Roan, E., Waters, C. M., Teng, B., Ghosh, M. & Schwingshackl, A. The 2-pore domain potassium channel TREK-1 regulates stretch-induced detachment of alveolar epithelial cells. PLoS ONE 9 , e89429. https://doi.org/10.1371/journal.pone.0089429 (2014).
Article ADS CAS PubMed PubMed Central Google Scholar
Schwingshackl, A., Teng, B., Ghosh, M. & Waters, C. M. Regulation of monocyte chemotactic protein-1 secretion by the two-pore-domain potassium (K2P) channel TREK-1 in human alveolar epithelial cells. Am. J. Transl. Res. 5 , 530–542 (2013).
CAS PubMed PubMed Central Google Scholar
Schwingshackl, A. et al. Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 302 , L93–L102. https://doi.org/10.1152/ajplung.00078.2011 (2012).
Schwingshackl, A. et al. Regulation of interleukin-6 secretion by the two-pore-domain potassium (K2P) channel Trek-1 in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. https://doi.org/10.1152/ajplung.00299.2012 (2012).
Honore, E. The neuronal background K2P channels: Focus on TREK1. Nat. Rev. Neurosci. 8 , 251–261. https://doi.org/10.1038/nrn2117 (2007).
Renigunta, V., Schlichthorl, G. & Daut, J. Much more than a leak: Structure and function of K(2)p-channels. Pflugers Arch. 467 , 867–894. https://doi.org/10.1007/s00424-015-1703-7 (2015).
Alloui, A. et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25 , 2368–2376. https://doi.org/10.1038/sj.emboj.7601116 (2006).
Xian Tao, L. et al. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 69 , 86–97. https://doi.org/10.1016/j.cardiores.2005.08.018 (2006).
Tomuschat, C. et al. Altered expression of a two-pore domain (K2P) mechano-gated potassium channel TREK-1 in Hirschsprung’s disease. Pediatr. Res. 80 , 729–733. https://doi.org/10.1038/pr.2016.140 (2016).
Hivelin, C. et al. Potentiation of calcium influx and insulin secretion in pancreatic beta cell by the specific TREK-1 blocker spadin. J. Diabetes Res. 2016 , 3142175. https://doi.org/10.1155/2016/3142175 (2016).
Reyes, R. et al. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 273 , 30863–30869 (1998).
Davis, K. A. & Cowley, E. A. Two-pore-domain potassium channels support anion secretion from human airway Calu-3 epithelial cells. Pflugers Arch. 451 , 631–641. https://doi.org/10.1007/s00424-005-1505-4 (2006).
Lembrechts, R. et al. Expression of mechanogated two-pore domain potassium channels in mouse lungs: Special reference to mechanosensory airway receptors. Histochem. Cell Biol. 136 , 371–385. https://doi.org/10.1007/s00418-011-0837-8 (2011).
Schwingshackl, A. et al. Deficiency of the two-pore-domain potassium channel TREK-1 promotes hyperoxia-induced lung injury. Crit. Care Med. 42 , e692-701. https://doi.org/10.1097/ccm.0000000000000603 (2014).
Schwingshackl, A. et al. Hyperoxia treatment of TREK-1/TREK-2/TRAAK-deficient mice is associated with a reduction in surfactant proteins. Am. J. Physiol. Lung Cell. Mol. Physiol. 313 , L1030-l1046. https://doi.org/10.1152/ajplung.00121.2017 (2017).
Lolicato, M. et al. K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature 547 , 364–368. https://doi.org/10.1038/nature22988 (2017).
Tertyshnikova, S. et al. BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: A putative potassium channel opener with bladder-relaxant properties. J. Pharmacol. Exp. Therap. 313 , 250–259. https://doi.org/10.1124/jpet.104.078592 (2005).
Veale, E. L. et al. Influence of the N terminus on the biophysical properties and pharmacology of TREK1 potassium channels. Mol. Pharmacol. 85 , 671–681. https://doi.org/10.1124/mol.113.091199 (2014).
Pope, L. et al. Protein and chemical determinants of BL-1249 action and selectivity for K2P channels. ACS Chem. Neurosci. 9 , 3153–3165. https://doi.org/10.1021/acschemneuro.8b00337 (2018).
Zhang, D., Gopalakrishnan, S. M., Freiberg, G. & Surowy, C. S. A thallium transport FLIPR-based assay for the identification of KCC2-positive modulators. J. Biomol. Screen 15 , 177–184. https://doi.org/10.1177/1087057109355708 (2010).
Wirtz, H. R. & Dobbs, L. G. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science (New York, N. Y.) 250 , 1266–1269 (1990).
Logan, M. R., Odemuyiwa, S. O. & Moqbel, R. Understanding exocytosis in immune and inflammatory cells: The molecular basis of mediator secretion. J. Allergy Clin. Immunol. 111 , 923–932 (2003) ( quiz 933 ).
Ramírez, D., Gonzalez, W., Fissore, R. A. & Carvacho, I. Conotoxins as tools to understand the physiological function of voltage-gated calcium (Ca(V)) channels. Mar. Drugs https://doi.org/10.3390/md15100313 (2017).
Hansen, P. B. et al. Functional importance of L- and P/Q-type voltage-gated calcium channels in human renal vasculature. Hypertension 58 , 464–470. https://doi.org/10.1161/hypertensionaha.111.170845 (2011).
Li, Z. et al. miR-16 inhibits hyperoxia-induced cell apoptosis in human alveolar epithelial cells. Mol. Med. Rep. 17 , 5950–5957. https://doi.org/10.3892/mmr.2018.8636 (2018).
Qin, S. et al. miR215p regulates type II alveolar epithelial cell apoptosis in hyperoxic acute lung injury. Mol. Med. Rep. 17 , 5796–5804. https://doi.org/10.3892/mmr.2018.8560 (2018).
Tamarapu Parthasarathy, P. et al. MicroRNA 16 modulates epithelial sodium channel in human alveolar epithelial cells. Biochem. Biophys. Res. Commun. 426 , 203–208. https://doi.org/10.1016/j.bbrc.2012.08.063 (2012).
Baumann, P. et al. Mechanical ventilation strategies alter cardiovascular biomarkers in an infant rat model. Physiol. Rep. https://doi.org/10.14814/phy2.13553 (2018).
Zinter, M. S. et al. Early plasma matrix metalloproteinase profiles: A novel pathway in pediatric acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.201804-0678OC (2018).
Bhargava, R. et al. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PLoS ONE 8 , e61405. https://doi.org/10.1371/journal.pone.0061405 (2013).
Minutti, C. M. et al. Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science (New York, N. Y.) 356 , 1076–1080. https://doi.org/10.1126/science.aaj2067 (2017).
O’Byrne, P. M. The demise of anti IL-5 for asthma, or not. Am. J. Respir. Crit. Care Med. 176 , 1059–1060. https://doi.org/10.1164/rccm.200708-1264ED (2007).
Bagnasco, D., Ferrando, M., Varricchi, G., Passalacqua, G. & Canonica, G. W. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int. Arch. Allergy Immunol. 170 , 122–131. https://doi.org/10.1159/000447692 (2016).
MacKenzie, G., Franks, N. P. & Brickley, S. G. Two-pore domain potassium channels enable action potential generation in the absence of voltage-gated potassium channels. Pflugers Arch. 467 , 989–999. https://doi.org/10.1007/s00424-014-1660-6 (2015).
Wiedmann, F. et al. N-glycosylation of TREK-1/hK(2P)2.1 two-pore-domain potassium (K(2P)) channels. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20205193 (2019).
Braun, G., Lengyel, M., Enyedi, P. & Czirjak, G. Differential sensitivity of TREK-1, TREK-2 and TRAAK background potassium channels to the polycationic dye ruthenium red. Br. J. Pharmacol. 172 , 1728–1738. https://doi.org/10.1111/bph.13019 (2015).
Maingret, F., Patel, A. J., Lesage, F., Lazdunski, M. & Honore, E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 274 , 26691–26696 (1999).
Kim, D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol. Sci. 24 , 648–654. https://doi.org/10.1016/j.tips.2003.10.008 (2003).
Schmidt, C. et al. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog. Biophys. Mol. Biol. 130 , 233–243. https://doi.org/10.1016/j.pbiomolbio.2017.05.004 (2017).
Perlis, R. H. et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: Association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology 33 , 2810–2819. https://doi.org/10.1038/npp.2008.6 (2008).
Decher, N. et al. Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 9 , 403–414. https://doi.org/10.15252/emmm.201606690 (2017).
Meduri, G. U. et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107 , 1062–1073 (1995).
van Zoelen, M. A. et al. Endogenous MCP-1 promotes lung inflammation induced by LPS and LTA. Mol. Immunol. 48 , 1468–1476. https://doi.org/10.1016/j.molimm.2011.04.001 (2011).
Mercer, P. F. et al. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 179 , 414–425. https://doi.org/10.1164/rccm.200712-1827OC (2009).
Zisman, D. A. et al. MCP-1 protects mice in lethal endotoxemia. J. Clin. Invest. 99 , 2832–2836. https://doi.org/10.1172/jci119475 (1997).
Narasaraju, T., Ng, H. H., Phoon, M. C. & Chow, V. T. MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am. J. Respir. Cell Mol. Biol. 42 , 732–743. https://doi.org/10.1165/rcmb.2008-0423OC (2010).
Van Der Wal, S. et al. Lidocaine increases the anti-inflammatory cytokine IL-10 following mechanical ventilation in healthy mice. Acta Anaesthesiol. Scand. 59 , 47–55. https://doi.org/10.1111/aas.12417 (2015).
Kurosaki, F. et al. AAV6-mediated IL-10 expression in the lung ameliorates bleomycin-induced pulmonary fibrosis in mice. Hum. Gene Ther. 29 , 1242–1251. https://doi.org/10.1089/hum.2018.024 (2018).
Swaroopa, D. et al. Association of serum interleukin-6, interleukin-8, and acute physiology and chronic health evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J. Crit. Care Med. 20 , 518–525. https://doi.org/10.4103/0972-5229.190369 (2016).
Andres-Hernando, A. et al. Circulating IL-6 upregulates IL-10 production in splenic CD4(+) T cells and limits acute kidney injury-induced lung inflammation. Kidney Int. 91 , 1057–1069. https://doi.org/10.1016/j.kint.2016.12.014 (2017).
Voiriot, G. et al. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir. Res. 18 , 64. https://doi.org/10.1186/s12931-017-0553-6 (2017).
Du, Y., Guo, M., Whitsett, J. A. & Xu, Y. ‘LungGENS’: A web-based tool for mapping single-cell gene expression in the developing lung. Thorax 70 , 1092–1094. https://doi.org/10.1136/thoraxjnl-2015-207035 (2015).
Hafner, S., Beloncle, F., Koch, A., Radermacher, P. & Asfar, P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann. Intensive Care 5 , 42. https://doi.org/10.1186/s13613-015-0084-6 (2015).
Janiszewski, J., Huizinga, J. D. & Blennerhassett, M. G. Mast cell ionic channels: Significance for stimulus-secretion coupling. Can. J. Physiol. Pharmacol. 70 , 1–7 (1992).
Liu, D., Zhang, J., Wu, J., Zhang, C. & Xu, T. Altered calcium-induced exocytosis in neutrophils from allergic patients. Int. Arch. Allergy Immunol. 134 , 281–287. https://doi.org/10.1159/000079165 (2004).
Zhang, R. Z., Yang, Q., Yim, A. P. & He, G. W. Alteration of cellular electrophysiologic properties in porcine pulmonary microcirculation after preservation with University of Wisconsin and Euro-Collins solutions. Ann. Thorac. Surgery 77 , 1944–1950. https://doi.org/10.1016/j.athoracsur.2003.11.051 (2004).
Herskovic, J. J., Speyer, C. L., Simples, J. E., Steffes, C. P. & Ram, J. L. Lipopolysaccharide (LPS) enhancement of outward current in lung pericytes. Lung 180 , 215–220. https://doi.org/10.1007/s004080000095 (2002).
Zharikov, S. I., Herrera, H. & Block, E. R. Role of membrane potential in hypoxic inhibition of L-arginine uptake by lung endothelial cells. Am. J. Physiol. 272 , L78-84. https://doi.org/10.1152/ajplung.1997.272.1.L78 (1997).
Lee, S. Y., Maniak, P. J., Ingbar, D. H. & O’Grady, S. M. Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane. Am. J. Physiol. Cell Physiol. 284 , C1614-1624. https://doi.org/10.1152/ajpcell.00429.2002 (2003).
Castranova, V., Jones, G. S. & Miles, P. R. Transmembrane potential of isolated rat alveolar type II cells. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 54 , 1511–1517. https://doi.org/10.1152/jappl.1983.54.6.1511 (1983).
Gallo, R. L., Finkelstein, J. N. & Notter, R. H. Characterization of the plasma and mitochondrial membrane potentials of alveolar type II cells by the use of ionic probes. Biochem. Biophys. Acta. 771 , 217–227. https://doi.org/10.1016/0005-2736(84)90536-4 (1984).
Falkenberg, C. V. & Jakobsson, E. A biophysical model for integration of electrical, osmotic, and pH regulation in the human bronchial epithelium. Biophys. J. 98 , 1476–1485. https://doi.org/10.1016/j.bpj.2009.11.045 (2010).
Knowles, M., Gatzy, J. & Boucher, R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N. Engl. J. Med. 305 , 1489–1495. https://doi.org/10.1056/nejm198112173052502 (1981).
Gaillard, E. A., Shaw, N. J., Wallace, H. L., Vince, G. & Southern, K. W. Electrical potential difference across the nasal epithelium is reduced in premature infants with chronic lung disease but is not associated with lower airway inflammation. Pediatr. Res. 61 , 77–82. https://doi.org/10.1203/01.pdr.0000250035.10339.ce (2007).
Ballke, E. H., Wiersbitzky, S., König, A. & Jährig, K. Transepithelial potential difference in the bronchial system of children with chronic bronchitis and bronchial asthma. Padiatr. Grenzgeb. 31 , 103–105 (1992).
Shlyonsky, V., Goolaerts, A., Mies, F. & Naeije, R. Electrophysiological characterization of rat type II pneumocytes in situ. Am. J. Respir. Cell Mol. Biol. 39 , 36–44. https://doi.org/10.1165/rcmb.2007-0227OC (2008).
Canella, R. et al. Involvement of the TREK-1 channel in human alveolar cell membrane potential and its regulation by inhibitors of the chloride current. J. Cell. Physiol. 234 , 17704–17713. https://doi.org/10.1002/jcp.28396 (2019).
Crago, P. E. & Makowski, N. S. Alteration of neural action potential patterns by axonal stimulation: The importance of stimulus location. J. Neural Eng. 11 , 056016. https://doi.org/10.1088/1741-2560/11/5/056016 (2014).
Article ADS PubMed PubMed Central Google Scholar
Fozzard, H. A. & Sheu, S. S. The resting potential in heart muscle. Adv. Myocardiol. 3 , 125–133. https://doi.org/10.1007/978-1-4899-5561-6_14 (1982).
Ma, C. et al. Hyperoxia causes mitochondrial fragmentation in pulmonary endothelial cells by increasing expression of pro-fission proteins. Arterioscler. Thromb. Vasc. Biol. 38 , 622–635. https://doi.org/10.1161/atvbaha.117.310605 (2018).
Kim, I., Donnelly, D. F. & Carroll, J. L. Postnatal hyperoxia impairs acute oxygen sensing of rat glomus cells by reduced membrane depolarization. Adv. Exp. Med. Biol. 758 , 49–54. https://doi.org/10.1007/978-94-007-4584-1_7 (2012).
Kim, I., Yang, D., Carroll, J. L. & Donnelly, D. F. Perinatal hyperoxia exposure impairs hypoxia-induced depolarization in rat carotid body glomus cells. Respir. Physiol. Neurobiol. 188 , 9–14. https://doi.org/10.1016/j.resp.2013.04.016 (2013).
Sousa, A. R. et al. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am. J. Respir. Cell Mol. Biol. 10 , 142–147. https://doi.org/10.1165/ajrcmb.10.2.8110469 (1994).
Paine, R. III. et al. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J. Immunol. 150 , 4561–4570 (1993).
Becker, S., Quay, J., Koren, H. S. & Haskill, J. S. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am. J. Physiol. 266 , L278-286. https://doi.org/10.1152/ajplung.1994.266.3.L278 (1994).
Lee, J. W. et al. NPS 2143, a selective calcium-sensing receptor antagonist inhibits lipopolysaccharide-induced pulmonary inflammation. Mol. Immunol. 90 , 150–157. https://doi.org/10.1016/j.molimm.2017.07.012 (2017).
Cordazzo, C. et al. Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca 2+ mobilization. Inflam. Res. 63 , 539–547. https://doi.org/10.1007/s00011-014-0723-7 (2014).
Proost, P., Wuyts, A. & Van Damme, J. Human monocyte chemotactic proteins-2 and -3: Structural and functional comparison with MCP-1. J. Leukoc. Biol. 59 , 67–74. https://doi.org/10.1002/jlb.59.1.67 (1996).
Gavrilin, M. A. et al. Site-directed mutagenesis of CCR2 identified amino acid residues in transmembrane helices 1, 2, and 7 important for MCP-1 binding and biological functions. Biochem. Biophys. Res. Commun. 327 , 533–540. https://doi.org/10.1016/j.bbrc.2004.12.037 (2005).
Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9 , 323–332. https://doi.org/10.1038/nrm2376 (2008).
Catterall, W. A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron 67 , 915–928. https://doi.org/10.1016/j.neuron.2010.08.021 (2010).
Murbartian, J., Lei, Q., Sando, J. J. & Bayliss, D. A. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J. Biol. Chem. 280 , 30175–30184. https://doi.org/10.1074/jbc.M503862200 (2005).
Salazar, E. & Knowles, J. H. An alaysis of pressure-volume characteristics of the lungs. J. Appl. Physiol. 19 , 97–104 (1964).
Messier, E. M., Mason, R. J. & Kosmider, B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp. Lung Res. 38 , 363–373. https://doi.org/10.3109/01902148.2012.713077 (2012).
Download references
Acknowledgements
We thank Dr. Michela Ottolia (UCLA) and her lab for ongoing discussions and their intellectual input. We also thank Dr. Daniel Minor (UCSF) for providing us with the ML335 compound and for sharing with us his knowledge about the pharmacokinetics and pharmacodynamics of the compound. This study was supported by the following Grants: NIH HL118118-3 (AS); NIH HL131526 (CMW); NIH HL134346 (RO).
Author information
Authors and affiliations.
Department of Pediatrics, University of California Los Angeles, 10833 Le Conte Ave, MDCC 12-475, Los Angeles, CA, 90095, USA
Tatiana Zyrianova, Benjamin Lopez, Leanne Wong, Victoria Nguyen, Sriharsha Talapaneni & Andreas Schwingshackl
Department of Anesthesiology and Perioperative Medicine, University of California Los Angeles, Los Angeles, CA, USA
Riccardo Olcese
Department of Physiology, University of California Los Angeles, Los Angeles, CA, USA
Department of Pulmonary and Critical Care Medicine, University of California Los Angeles, Los Angeles, CA, USA
John Belperio
Department of Physiology, University of Kentucky, Lexington, KY, USA
Christopher M. Waters
You can also search for this author in PubMed Google Scholar
Contributions
T.Z.: experimental design and execution, manuscript writing and editing. B.L.: experimental design and execution. R.O.: experimental design and manuscript editing. J.B.: experimental design and manuscript editing. C.M.W.: experimental design and manuscript editing. L.W.: experimental execution. V.N.: experimental execution. S.T.: experimental execution. A.S.: experimental design, manuscript writing and editing.
Corresponding author
Correspondence to Andreas Schwingshackl .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary legends., supplementary figure 1., supplementary figure 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zyrianova, T., Lopez, B., Olcese, R. et al. K 2P 2.1 (TREK-1) potassium channel activation protects against hyperoxia-induced lung injury. Sci Rep 10 , 22011 (2020). https://doi.org/10.1038/s41598-020-78886-y
Download citation
Received : 24 June 2020
Accepted : 01 December 2020
Published : 15 December 2020
DOI : https://doi.org/10.1038/s41598-020-78886-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Salidroside attenuates hali via il-17a-mediated ferroptosis of alveolar epithelial cells by regulating act1-traf6-p38 mapk pathway.
- Zhongfu Zuo
- Zhansheng Hu
Cell Communication and Signaling (2022)
Emerging roles of mechanosensitive ion channels in acute lung injury/acute respiratory distress syndrome
- Zhiqiang Hu
Respiratory Research (2022)
Mechanism of Adipose-Derived Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying miR-21-5p in Hyperoxia-Induced Lung Injury
- Zhihui Zhang
Stem Cell Reviews and Reports (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
TREK-1, a K+ channel involved in polymodal pain perception
Affiliation.
- 1 Laboratoire de Pharmacologie Médicale EA 3848 INSERM/Faculté de Médecine/CHU, Clermont-Ferrand, France.
- PMID: 16675954
- PMCID: PMC1478167
- DOI: 10.1038/sj.emboj.7601116
The TREK-1 channel is a temperature-sensitive, osmosensitive and mechano-gated K+ channel with a regulation by Gs and Gq coupled receptors. This paper demonstrates that TREK-1 qualifies as one of the molecular sensors involved in pain perception. TREK-1 is highly expressed in small sensory neurons, is present in both peptidergic and nonpeptidergic neurons and is extensively colocalized with TRPV1, the capsaicin-activated nonselective ion channel. Mice with a disrupted TREK-1 gene are more sensitive to painful heat sensations near the threshold between anoxious warmth and painful heat. This phenotype is associated with the primary sensory neuron, as polymodal C-fibers were found to be more sensitive to heat in single fiber experiments. Knockout animals are more sensitive to low threshold mechanical stimuli and display an increased thermal and mechanical hyperalgesia in conditions of inflammation. They display a largely decreased pain response induced by osmotic changes particularly in prostaglandin E2-sensitized animals. TREK-1 appears as an important ion channel for polymodal pain perception and as an attractive target for the development of new analgesics.
Publication types
- Research Support, Non-U.S. Gov't
- Ganglia, Spinal / cytology
- In Situ Hybridization
- Mice, Knockout
- Nerve Fibers, Unmyelinated / metabolism
- Neurons, Afferent / cytology
- Neurons, Afferent / metabolism
- Nociceptors / metabolism*
- Pain / metabolism*
- Pain Measurement
- Patch-Clamp Techniques
- Perception / physiology*
- Potassium Channels, Tandem Pore Domain / genetics
- Potassium Channels, Tandem Pore Domain / metabolism*
- RNA, Messenger / metabolism
- Potassium Channels, Tandem Pore Domain
- RNA, Messenger
- potassium channel protein TREK-1
The Future of ‘Star Trek’: From ‘Starfleet Academy’ to New Movies and Michelle Yeoh, How the 58-Year-Old Franchise Is Planning for the Next Generation of Fans
“I can’t believe I get to play the captain of the Enterprise.”
“Strange New Worlds” is the 12th “Star Trek” TV show since the original series debuted on NBC in 1966, introducing Gene Roddenberry’s vision of a hopeful future for humanity. In the 58 years since, the “Star Trek” galaxy has logged 900 television episodes and 13 feature films, amounting to 668 hours — nearly 28 days — of content to date. Even compared with “Star Wars” and the Marvel Cinematic Universe, “Star Trek” stands as the only storytelling venture to deliver a single narrative experience for this long across TV and film.
In other words, “Star Trek” is not just a franchise. As Alex Kurtzman , who oversees all “Star Trek” TV production, puts it, “‘Star Trek’ is an institution.”
Without a steady infusion of new blood, though, institutions have a way of fading into oblivion (see soap operas, MySpace, Blockbuster Video). To keep “Star Trek” thriving has meant charting a precarious course to satisfy the fans who have fueled it for decades while also discovering innovative ways to get new audiences on board.
“Doing ‘Star Trek’ means that you have to deliver something that’s entirely familiar and entirely fresh at the same time,” Kurtzman says.
The franchise has certainly weathered its share of fallow periods, most recently after “Nemesis” bombed in theaters in 2002 and UPN canceled “Enterprise” in 2005. It took 12 years for “Star Trek” to return to television with the premiere of “Discovery” in 2017; since then, however, there has been more “Star Trek” on TV than ever: The adventure series “Strange New Worlds,” the animated comedy “Lower Decks” and the kids series “Prodigy” are all in various stages of production, and the serialized thriller “Picard” concluded last year, when it ranked, along with “Strange New Worlds,” among Nielsen’s 10 most-watched streaming original series for multiple weeks. Nearly one in five Paramount+ subscribers in the U.S. is watching at least one “Star Trek” series, according to the company, and more than 50% of fans watching one of the new “Trek” shows also watch at least two others. The new shows air in 200 international markets and are dubbed into 35 languages. As “Discovery” launches its fifth and final season in April, “Star Trek” is in many ways stronger than it’s ever been.
“’Star Trek’s fans have kept it alive more times than seems possible,” says Eugene Roddenberry, Jr., who executive produces the TV series through Roddenberry Entertainment. “While many shows rightfully thank their fans for supporting them, we literally wouldn’t be here without them.”
But the depth of fan devotion to “Star Trek” also belies a curious paradox about its enduring success: “It’s not the largest fan base,” says Akiva Goldsman, “Strange New Worlds” executive producer and co-showrunner. “It’s not ‘Star Wars.’ It’s certainly not Marvel.”
When J.J. Abrams rebooted “Star Trek” in 2009 — with Chris Pine, Zachary Quinto and Zoe Saldaña playing Kirk, Spock and Uhura — the movie grossed more than any previous “Star Trek” film by a comfortable margin. But neither that film nor its two sequels broke $500 million in global grosses, a hurdle every other top-tier franchise can clear without breaking a sweat.
There’s also the fact that “Star Trek” fans are aging. I ask “The Next Generation” star Jonathan Frakes, who’s acted in or directed more versions of “Star Trek” than any other person alive, how often he meets fans for whom the new “Star Trek” shows are their first. “Of the fans who come to talk to me, I would say very, very few,” he says. “‘Star Trek’ fans, as we know, are very, very, very loyal — and not very young.”
As Stapf puts it: “There’s a tried and true ‘Trek’ fan that is probably going to come to every ‘Star Trek,’ no matter what it is — and we want to expand the universe.”
Every single person I spoke to for this story talked about “Star Trek” with a joyful earnestness as rare in the industry as (nerd alert) a Klingon pacifist.
“When I’m meeting fans, sometimes they’re coming to be confirmed, like I’m kind of a priest,” Ethan Peck says during a break in filming on the “Strange New Worlds” set. He’s in full Spock regalia — pointy ears, severe eyebrows, bowl haircut — and when asked about his earliest memories of “Star Trek,” he stares off into space in what looks like Vulcan contemplation. “I remember being on the playground in second or third grade and doing the Vulcan salute, not really knowing where it came from,” he says. “When I thought of ‘Star Trek,’ I thought of Spock. And now I’m him. It’s crazy.”
To love “Star Trek” is to love abstruse science and cowboy diplomacy, complex moral dilemmas and questions about the meaning of existence. “It’s ultimately a show with the most amazing vision of optimism, I think, ever put on-screen in science fiction,” says Kurtzman, who is 50. “All you need is two minutes on the news to feel hopeless now. ‘Star Trek’ is honestly the best balm you could ever hope for.”
I’m getting a tour of the USS Enterprise from Scotty — or, rather, “Strange New World” production designer Jonathan Lee, who is gushing in his native Scottish burr as we step into the starship’s transporter room. “I got such a buzzer from doing this, I can’t tell you,” he says. “I actually designed four versions of it.”
Lee is especially proud of the walkway he created to run behind the transporter pads — an innovation that allows the production to shoot the characters from a brand-new set of angles as they beam up from a far-flung planet. It’s one of the countless ways that this show has been engineered to be as cinematic as possible, part of Kurtzman’s overall vision to make “Star Trek” on TV feel like “a movie every week.”
Kurtzman’s tenure with “Star Trek” began with co-writing the screenplay for Abrams’ 2009 movie, which was suffused with a fast-paced visual style that was new to the franchise. When CBS Studios approached Kurtzman in the mid-2010s about bringing “Star Trek” back to TV, he knew instinctively that it needed to be just as exciting as that film.
“The scope was so much different than anything we had ever done on ‘Next Gen,’” says Frakes, who’s helmed two feature films with the “Next Generation” cast and directed episodes of almost every live-action “Trek” TV series, including “Discovery” and “Strange New Worlds.” “Every department has the resources to create.”
A new science lab set for Season 3, for example, boasts a transparent floor atop a four-foot pool of water that swirls underneath the central workbench, and the surrounding walls sport a half dozen viewscreens with live schematics custom designed by a six-person team. “I like being able to paint on a really big canvas,” Kurtzman says. “The biggest challenge is always making sure that no matter how big something gets, you’re never losing focus on that tiny little emotional story.”
At this point, is there a genre that “Strange New Worlds” can’t do? “As long as we’re in storytelling that is cogent and sure handed, I’m not sure there is,” Goldsman says with an impish smile. “Could it do Muppets? Sure. Could it do black and white, silent, slapstick? Maybe!”
This approach is also meant to appeal to people who might want to watch “Star Trek” but regard those 668 hours of backstory as an insurmountable burden. “You shouldn’t have to watch a ‘previously on’ to follow our show,” Myers says.
To achieve so many hairpin shifts in tone and setting while maintaining Kurtzman’s cinematic mandate, “Strange New Worlds” has embraced one of the newest innovations in visual effects: virtual production. First popularized on the “Star Wars” series “The Mandalorian,” the technology — called the AR wall — involves a towering circular partition of LED screens projecting a highly detailed, computer-generated backdrop. Rather than act against a greenscreen, the actors can see whatever fantastical surroundings their characters are inhabiting, lending a richer level of verisimilitude to the show.
But there is a catch. While the technology is calibrated to maintain a proper sense of three-dimensional perspective through the camera lens, it can be a bit dizzying for anyone standing on the set. “The images on the walls start to move in a way that makes no sense,” says Mount. “You end up having to focus on something that’s right in front of you so you don’t fall down.”
And yet, even as he’s talking about it, Mount can’t help but break into a boyish grin. “Sometimes we call it the holodeck,” he says. In fact, the pathway to the AR wall on the set is dotted with posters of the virtual reality room from “The Next Generation” and the words “Enter Holodeck” in a classic “Trek” font.
“I want to take one of those home with me,” Peck says. Does the AR wall also affect him? “I don’t really get disoriented by it. Spock would not get ill, so I’m Method acting.”
I’m on the set of the “Star Trek” TV movie “Section 31,” seated in an opulent nightclub with a view of a brilliant, swirling nebula, watching Yeoh rehearse with director Olatunde Osunsanmi and her castmates. Originally, the project was announced as a TV series centered on Philippa Georgiou, the semi-reformed tyrant Yeoh originated on “Discovery.” But between COVID delays and the phenomenon of “Everything Everywhere All at Once,” there wasn’t room in the veteran actress’s schedule to fit a season of television. Yeoh was undaunted.
“We’d never let go of her,” she says of her character. “I was just blown away by all the different things I could do with her. Honestly, it was like, ‘Let’s just get it done, because I believe in this.’”
If that means nothing to you, don’t worry: The enormity of the revelation that Garrett is being brought back is meant only for fans. If you don’t know who the character is, you’re not missing anything.
“It was always my goal to deliver an entertaining experience that is true to the universe but appeals to newcomers,” says screenwriter Craig Sweeny. “I wanted a low barrier of entry so that anybody could enjoy it.”
Nevertheless, including Garrett on the show is exactly the kind of gasp-worthy detail meant to flood “Star Trek” fans with geeky good feeling.
“You cannot create new fans to the exclusion of old fans,” Kurtzman says. “You must serve your primary fan base first and you must keep them happy. That is one of the most important steps to building new fans.”
On its face, that maxim would make “Section 31” a genuine risk. The titular black-ops organization has been controversial with “Star Trek” fans since it was introduced in the 1990s. “The concept is almost antagonistic to some of the values of ‘Star Trek,’” Sweeny says. But he still saw “Section 31” as an opportunity to broaden what a “Star Trek” project could be while embracing the radical inclusivity at the heart of the franchise’s appeal.
“Famously, there’s a spot for everybody in Roddenberry’s utopia, so I was like, ‘Well, who would be the people who don’t quite fit in?’” he says. “I didn’t want to make the John le Carré version, where you’re in the headquarters and it’s backbiting and shades of gray. I wanted to do the people who were at the edges, out in the field. These are not people who necessarily work together the way you would see on a ‘Star Trek’ bridge.”
For Osunsanmi, who grew up watching “The Next Generation” with his father, it boils down to a simple question: “Is it putting good into the world?” he asks. “Are these characters ultimately putting good into the world? And, taking a step back, are we putting good into the world? Are we inspiring humans watching this to be good? That’s for me what I’ve always admired about ‘Star Trek.’”
Should “Section 31” prove successful, Yeoh says she’s game for a sequel. And Kurtzman is already eyeing more opportunities for TV movies, including a possible follow-up to “Picard.” The franchise’s gung-ho sojourn into streaming movies, however, stands in awkward contrast to the persistent difficulty Paramount Pictures and Abrams’ production company Bad Robot have had making a feature film following 2016’s “Star Trek Beyond” — the longest theaters have gone without a “Star Trek” movie since Paramount started making them.
First, a movie reuniting Pine’s Capt. Kirk with his late father — played in the 2009 “Star Trek” by Chris Hemsworth — fell apart in 2018. Around the same time, Quentin Tarantino publicly flirted with, then walked away from, directing a “Star Trek” movie with a 1930s gangster backdrop. Noah Hawley was well into preproduction on a “Star Trek” movie with a brand-new cast, until then-studio chief Emma Watts abruptly shelved it in 2020. And four months after Abrams announced at Paramount’s 2022 shareholders meeting that his 2009 cast would return for a movie directed by Matt Shakman (“WandaVision”), Shakman left the project to make “The Fantastic Four” for Marvel. (It probably didn’t help that none of the cast had been approached before Abrams made his announcement.)
The studio still intends to make what it’s dubbed the “final chapter” for the Pine-Quinto-Saldaña cast, and Steve Yockey (“The Flight Attendant”) is writing a new draft of the script. Even further along is another prospective “Star Trek” film written by Seth Grahame-Smith (“Abraham Lincoln: Vampire Hunter”) and to be directed by Toby Haynes (“Andor,” “Black Mirror: USS Callister”) that studio insiders say is on track to start preproduction by the end of the year. That project will serve as an origin story of sorts for the main timeline of the entire franchise. In both cases, the studio is said to be focused on rightsizing the budgets to fit within the clear box office ceiling for “Star Trek” feature films.
Far from complaining, everyone seems to relish the challenge. Visual effects supervisor Jason Zimmerman says that “working with Alex, the references are always at least $100 million movies, if not more, so we just kind of reverse engineer how do we do that without having to spend the same amount of money and time.”
The workload doesn’t seem to faze him either. “Visual effects people are a big, big ‘Star Trek’ fandom,” he says. “You naturally just get all these people who go a little bit above and beyond, and you can’t trade that for anything.”
In one of Kurtzman’s several production offices in Toronto, he and production designer Matthew Davies are scrutinizing a series of concept drawings for the newest “Star Trek” show, “Starfleet Academy.” A bit earlier, they showed me their plans for the series’ central academic atrium, a sprawling, two-story structure that will include a mess hall, amphitheater, trees, catwalks, multiple classrooms and a striking view of the Golden Gate Bridge in a single, contiguous space. To fit it all, they plan to use every inch of Pinewood Toronto’s 45,900 square foot soundstage, the largest in Canada.
But this is a “Star Trek” show, so there do need to be starships, and Kurtzman is discussing with Davies about how one of them should look. The issue is that “Starfleet Academy” is set in the 32nd century, an era so far into the future Kurtzman and his team need to invent much of its design language.
“For me, this design is almost too Klingon,” Kurtzman says. “I want to see the outline and instinctively, on a blink, recognize it as a Federation ship.”
The time period was first introduced on Season 3 of “Discovery,” when the lead character, Michael Burnham (Sonequa Martin-Green), transported the namesake starship and its crew there from the 23rd century. “It was exciting, because every time we would make a decision, we would say, ‘And now that’s canon,’” says Martin-Green.
“We listened to a lot of it,” Kurtzman says. “I think I’ve been able to separate the toxic fandom from really true fans who love ‘Star Trek’ and want you to hear what they have to say about what they would like to see.”
By Season 2, the “Discovery” writers pivoted from its dour, war-torn first season and sent the show on its trajectory 900-plus years into the future. “We had to be very aware of making sure that Spock was in the right place and that Burnham’s existence was explained properly, because she was never mentioned in the original series,” says executive producer and showrunner Michelle Paradise. “What was fun about jumping into the future is that it was very much fresh snow.”
That freedom affords “Starfleet Academy” far more creative latitude while also dramatically reducing how much the show’s target audience of tweens and teens needs to know about “Star Trek” before watching — which puts them on the same footing as the students depicted in the show. “These are kids who’ve never had a red alert before,” Noga Landau, executive producer and co-showrunner, says. “They never had to operate a transporter or be in a phaser fight.”
In the “Starfleet Academy” writers’ room in Secret Hideout’s Santa Monica offices, Kurtzman tells the staff — a mix of “Star Trek” die-hards, part-time fans and total newbies — that he wants to take a 30,000-foot view for a moment. “I think we need to ground in science more throughout the show,” he says, a giant framed photograph of Spock ears just over his shoulder. “The kids need to use science more to solve problems.”
Immediately, one of the writers brightens. “Are you saying we can amp up the techno-babble?” she says. “I’m just excited I get to use my computer science degree.”
After they break for lunch, Kurtzman is asked how much longer he plans to keep making “Star Trek.”
“The minute I fall out of love with it is the minute that it’s not for me anymore. I’m not there yet,” he says. “To be able to build in this universe to tell stories that are fundamentally about optimism and a better future at a time when the world seems to be falling apart — it’s a really powerful place to live every day.”
More From Our Brands
Gop rep. turner: republicans have ‘uttered’ russian propaganda ‘on the house floor’, how todd snyder is decoding luxury menswear for a new generation, purdue, uconn advance as host committee seeks another final four, the best loofahs and body scrubbers, according to dermatologists, march madness 2024: how to watch iowa vs. south carolina in women’s national championship, verify it's you, please log in.

Yet another Star Trek channel has been added to Pluto TV
S tar Trek fans have not had to go far to find places to find the best episodes the franchise has ever produced. The television channels, MeTV and Heroes and Icons, both have blocks dedicated to the classic franchise, with Star Trek's original series getting play on both channels, and The Next Generation, Voyager, Deep Space Nine, and Enterprise getting near-daily blocks dedicated to it on Heroes and Icons.
That's not all, however, if you're a Star Trek fan who wants more than a Saturday night experience on MeTV or wants more than just an hour of a specific series six nights a week on Heroes and Icons, there's always the streaming service Pluto TV.
The free streaming service featured the original series, The Next Generation, Voyager, and Deep Space Nine across two channels, but due to the popularity of Deep Space Nine on the service, a Deep Space Nine channel will now mark the third Star Trek streaming channel for the service. Not only that, but it's the first channel with a dedicated show.
The Star Trek channel features the original series and The Next Generation while the More Star Trek channel features Voyager and Deep Space Nine, though now it's likely to focus solely on Voyager since Deep Space Nine has its own channel.
It's likely that the goal is to get a fourth channel and have the original series, The Next Generation, Voyager, and Deep Space Nine all with their own specific channel. Not only that but there are specific seasons of each of the four shows available to watch for free on-demand.
As of right now, the only series from the golden era to not feature at all in any way on the service is the Scott Bakula-backed series , Star Trek: Enterprise, though it seems like that shows addition is only a matter of time.
This article was originally published on redshirtsalwaysdie.com as Yet another Star Trek channel has been added to Pluto TV .

Screen Rant
Star trek: discovery season 5 brings back enterprise captain archer tribute.
The spirit of Star Trek: Enterprise's Captain Jonathan Archer continues to be felt in Star Trek: Discovery season 5 over a thousand years later.
Warning: SPOILERS for Star Trek: Discovery Season 5, Episode 1 - "Red Directive"
- Season 5 of Star Trek: Discovery pays tribute to Captain Archer from Star Trek: Enterprise.
- Archer Space Dock in Discovery serves as a hub for Starfleet upgrades and new starship construction in honor of Jonathan Archer.
- Captain Archer's legacy and impact on the formation of the Federation are essential to Star Trek: Discovery's future.
Star Trek: Discovery season 5 brought back the 32nd century Starfleet's tribute to Captain Jonathan Archer (Scott Bakula) from Star Trek: Enterprise. Star Trek: Discovery and Star Trek: Enterprise are TV series at opposite points bookending Star Trek 's Prime Universe timeline . Enterprise is set in the 22nd century and charts the pioneering voyages of the NX-01, the first Starship Enterprise commanded by Captain Archer. Star Trek: Discovery seasons 3-5 are set over a thousand years later in the 32nd century.
Star Trek: Discovery season 1 began in 2256, a century after Captain Archer's NX-01 Enterprise first set off to explore the galaxy. Although Captain Michael Burnham (Sonequa Martin-Green) and the USS Discovery's crew never met Archer, Star Trek: Discovery certainly bore the influence of Star Trek: Enterprise , especially in Discovery 's early seasons. Both series were Star Trek prequels, and Discovery 's original blue Starfleet uniforms were a visual link to the distinctive blue jumpsuits worn on Star Trek: Enterprise by Captain Archer's crew .
Star Trek: Discovery Season 5 Returning Cast & New Character Guide
Star trek: discovery brings back archer space dock, captain archer's spirit is part of the 32nd-century starfleet.
Star Trek: Discovery season 5's premiere , "Red Directive," saw the return of the Archer Space Dock. Following a mission to Q'Mau where Captain Michael Burnham, Captain Rayner (Callum Keith Rennie), and Cleveland Booker (David Ajala) were unable to apprehend couriers Moll (Eve Harlow) and L'ak (Elias Toufexis), the USS Discovery and USS Antares used their combined shields to protect a Q'Mau settlement from an avalanche. Afterward, a dusty Discovery jumped back for repairs at the Archer Space Dock near United Federation of Planets headquarters.
The goal of the Archer Space Dock is to upgrade the existing Starfleet and build the next generation of starships.
The Archer Space Dock was introduced in Star Trek: Discovery season 4's premiere, "Kobayashi Maru." The facility was unveiled by Federation President Laira Rillak (Chelah Horsdal) to the first new class of Starfleet Academy. The goal of the Archer Space Dock is to upgrade the existing Starfleet and build the next generation of starships. It was only fitting to name the Archer Space Dock after the Captain of the first Starship Enterprise, and the first Federation President, Jonathan Archer .
The Archer Space Dock is reminiscent of previous facilities that have built and repaired the USS Enterprise throughout Star Trek .
Enterprise’s Archer Is An Important Part Of Star Trek: Discovery Millennium Celebration
The federation began with archer a thousand years ago (give or take a few decades).
Although Captain Jonathan Archer wasn't name-dropped like Star Trek: The Next Generation 's Captain Jean-Luc Picard (Patrick Stewart) was in Star Trek: Discovery season 5's premiere, Archer's spirit was evoked in the Federation's Millennium Celebration . Discovery season 5 is set in 3191, a thousand and 30 years after the founding of the United Federation of Planets . Captain Archer was a pivotal figure whose voyages forged the bonds between United Earth, Vulcan, Tellar Prime, and Andoria that led to the formation of the Federation.
Captain Archer was aware of events in the 30th century as a result of his involvement in the Temporal War in Star Trek: Enterprise.
Jonathan Archer is essentially the George Washington of the Federation . After his decade-long run as Captain of the Enterprise, Archer served as the first Federation President. There would be no Federation without Jonathan Archer, and he would be pleased to know that the Federation still endures in the 32nd century. Star Trek: Discovery is forging the future in the 32nd century, but the Archer Space Dock shows the Federation never forgets it was Captain Jonathan Archer who helped it begin.
Star Trek: Discovery and Star Trek: Enterprise are streaming on Paramount+
- Reference Manager
- Simple TEXT file

People also looked at
Mini review article, trek channels in mechanotransduction: a focus on the cardiovascular system.

- 1 Laboratory of Neuroscience, CINBIO, University of Vigo, Vigo, Spain
- 2 Laboratory of Neuroscience, Galicia Sur Health Research Institute (IISGS), Vigo, Spain
Mechano-electric feedback is one of the most important subsystems operating in the cardiovascular system, but the underlying molecular mechanism remains rather unknown. Several proteins have been proposed to explain the molecular mechanism of mechano-transduction. Transient receptor potential (TRP) and Piezo channels appear to be the most important candidates to constitute the molecular mechanism behind of the inward current in response to a mechanical stimulus. However, the inhibitory/regulatory processes involving potassium channels that operate on the cardiac system are less well known. TWIK-Related potassium (TREK) channels have emerged as strong candidates due to their capacity for the regulation of the flow of potassium in response to mechanical stimuli. Current data strongly suggest that TREK channels play a role as mechano-transducers in different components of the cardiovascular system, not only at central (heart) but also at peripheral (vascular) level. In this context, this review summarizes and highlights the main existing evidence connecting this important subfamily of potassium channels with the cardiac mechano-transduction process, discussing molecular and biophysical aspects of such a connection.
Introduction
While great strides have been made in understanding the molecular mechanisms of touch ( 1 – 3 ), cardiovascular mechanotransduction remains a complex and enigmatic process that is not yet fully understood. This review highlights the critical importance of mechano-sensitivity in maintaining proper cardiovascular function, and the challenges associated with elucidating its underlying mechanisms.
Multiple elements can sense the different mechanical forces affecting the cellular body, for example, elements of the extracellular matrix such as integrins, elements of the cytoskeleton, G-protein-coupled receptors or different ion channels ( 4 ). This review is focused on TREK channels, a subfamily of the two-pore-domain potassium (K2P) channels encoded by genes named KCNK, which are capable of detecting mechanical stimuli altering their opening and closing kinetics. These mechano-sensitive ion channels are membrane proteins that allow cells to respond and adapt to physical forces ( 5 ), playing a crucial role in mechano-transduction processes ( 6 , 7 ). Mechanical forces are fundamental in cardiovascular biology, however, the mechanisms that support this physiological process have yet to be elucidated. In this sense, the link between electrical stimulation and mechanical contractions is widely established, and the mechanism by which an electrical stimulus produces muscle contraction is widely accepted ( 8 ). On the contrary, the process by which mechanical forces can influence the electrical properties (mechano-electric feedback) of the cardiovascular cells is still poorly understood ( 9 , 10 ). Mechano-electric feedback is one of the most important subsystems that operate within the cardiovascular system ( 11 ), it can be defined as the process by which mechanical stimuli are converted into electrical signals and plays a key role in the functioning of cardiovascular homeostasis ( 2 , 12 , 13 ). In the heart, different mechano-sensitive structures have been identified, with myocytes being the most relevant ( 14 ), while at peripheral level, smooth muscle fibers (present in veins and arteries) are the main elements.
Roughly speaking (without considering chloride channels) ion channels can be separated into two categories. When activated, certain channels, regardless of their selectivity, can either depolarize or hyperpolarize the cell membrane. Applying this idea to the mechanobiology context, these families are known as depolarizing non-selective cationic channels and hyperpolarizing potassium selective channels. In this context, TRP and Piezo channels are part of the first category. They are a nonselective Na + , Ca 2+ (among others) conductors. TRP channels are usually considered as dominant elements in mechano-sensitivity ( 15 ) and they are part of the mechanosensitive non-selective cardiac current family ( 16 – 19 ). However, they have been shown to be insensitive to membrane stretch ( 20 ) and are not considered primary mechanotransducers ( 21 ). Piezo channels are also considered to be transducers of mechanical stimuli and are widely expressed in the cardiovascular system ( 22 ). and they could work like baroreceptors ( 23 ) even during cardiac development ( 24 ). The second category is made up of TREK channels (TREK-1, TREK-2 and TRAAK) and they are probably the only mechanically-gated potassium channels playing an important role in the process of mechanical transduction ( 25 ). Given their widespread expression throughout the cardiovascular system ( 26 ), these channels are emerging as potential contributors to cardiac mechano-electrical feedback and mechano-associated pathologies. Thus, we reviewed the evidence supporting this possibility.
Mechano-regulation of TREK channels
MS ion channels can be activated by two different mechanisms. The mechanism called tethering needs several cytoskeletal proteins as scaffold proteins to activate the mechano-sensor, this is the case of TRP channels ( 27 ). The other mechanism implies the activation of the channels by the tension in the bilayer itself, without the need for other cellular structures, in this group are TREK channels, see ( 28 ) for controversial.
The molecular mechanism underlying the sensitivity of TREKs to membrane deformation induced by mechanical forces has been extensively investigated, stating that cellular integrity is not essential for mechanical channel activation ( 7 , 29 ), indeed TREK channels are regulated by a mechanism called “selective filter” ( 30 , 31 ) (see Figure 1 ). This consists in a change of conformation in the narrow zone of the pore that regulates the flow of ions, similar to the C-type blockade studied in voltage-dependent potassium channels such as inward rectifier potassium (Kir) channels ( 34 , 35 ). It has been shown that with the pore closed, the helical protein structures would not interfere with the passage of ions ( 36 ), contrary to what occurs in most potassium channels ( 37 – 39 ). Although this selective filter mechanism is widely accepted ( 36 , 40 , 41 ), there are still many open questions ( 42 ) and other gating mechanisms could be present and activated depending on the stimulus ( 43 , 44 ).
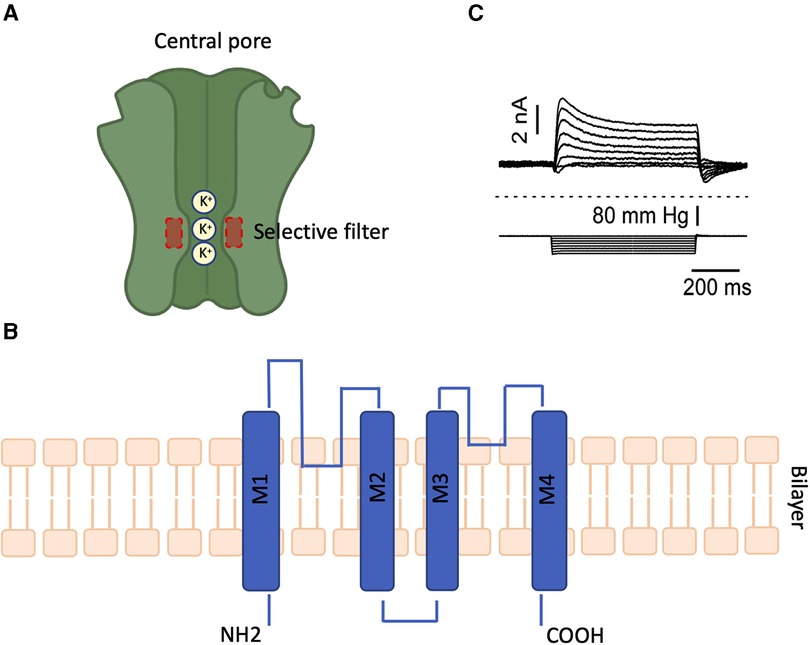
Figure 1 . ( A ) view of the TREK structure in conventional configuration. Red dotted lines indicate the selective filter. ( B ) Topological model proposed for K2P channels, each subunit has two pore forming domain (P loops) and four transmembrane domains (denoted M1-M4). ( C ) Representative response to a mechanical stimuli of TREK showing that it has minimal desensitization in the inside-out configuration. Adapted from ( 32 , 33 ).
Two states, called “Up” and “Down”, have been described for TREK channels and although in both states the pore is open, it has been suggested that only the Up state can be considered conductive and that in the Down one the conductivity is residual ( 36 , 45 ). It has been shown that TREK channels can sense mechanical forces directly through the bilayer and it has been demonstrated that TREK channels have located the mechano-gate in the selectivity filter ( 46 , 47 ). Thus when the membrane is stretched there is a conformational change in the channel's selective filter that favors the entry into the Up state, more conductive when compared with the Down state, notwithstanding this theory has generated some controversy ( 48 ). As mentioned above, two mechanisms enable channels to perceive mechanical forces: direct (Piezo and TREK channels) and indirect (TRP channels). In addition to experimental conditions, while the mechanism underlying the mechanosensitivity of TRP channels is well-established ( 17 , 21 , 27 ), it is apparent that membrane deformation can also bring about mechanical changes in different cytoskeleton proteins, which can contribute to the feedback of tension in the bilayer. Therefore, these mechanisms may not be entirely separate and could potentially complement each other under physiological conditions. For instance, some studies have demonstrated that Piezos are solely responsive to shear stress (frictional force) ( 49 , 50 ), but not to stretch. Furthermore, Piezos can interact with other MS ion channels like TRP channels ( 51 , 52 ) which may conceal their behaviour under certain experimental conditions, leading to further variability.
Role of TREK channels in the cardiovascular system
As we have recently reviewed, TREK-1 is the most expressed TREK channel in heart, both in neuronal and non-neuronal tissue, including the sinoatrial node, cardiomyocytes and purkinje fibers ( 26 ). Several studies have shown how TREK-1 is extensively expressed in heart using molecular techniques, including qRT-PCR and WB ( 53 , 54 ). Confocal imaging also showed TREK-1 arranged in longitudinal stripes at the surface of the cardiomyocytes in rats ( 55 ). Consistently, whole-cell patch-clamp electrophysiological recordings have shown clearly the presence of a potassium current conducted by TREK channels in cardiac cells in both murine and human ( 56 – 58 ). In summary, the presence of TREK-1 in the heart tissue of various mammals including rodents and humans has been widely demonstrated ( Table 1 ). However, the other two members of the TREK subfamily (TREK-2 and TRAAK) have been poorly localized ( 32 , 64 – 68 ).
Table 1 . Non-systematic but representative summary of the presence of TREK channels in the cardiovascular system.
From a functional point of view, TREK-1 plays a critical role in countering the depolarizing effect of mechano-activated cationic currents, contributing to stimulation-activated central (heart) feedback mechanics in the cardiovascular system ( 69 ). TREK-1 channels also have a potential role in regulating the normal activity of sinoatrial node-hosted pacemakers by preventing the occurrence of ventricular extrasystoles ( 55 , 70 ). Inhibition of TREK-1 channels via PKA during sympathetic stimulation may decrease transmural dispersion of repolarization and prevent the occurrence of arrhythmias ( 58 ), indicating that TREK-1 may have an essential function in the cardiac conduction system ( 71 ). In cardiomyocytes, the refractory period is critical in preventing premature excitation and arrhythmias. The duration or amplitude of the action potential depends on a delicate balance between inward-potassium and outward currents during the action potential plateau. TREK-1, as well as BKCa (large conductance K + channel, both voltage and calcium-gated) or KATP (ATP-sensitive potassium) channels, are the main candidates encoding the cardiac stretch-activated potassium current ( 72 ). However, in contrast to TREK-1, in the human heart, BKCa and KATP channels are poorly expressed, making TREK-1 the primary candidate for encoding cardiac stretch-activated potassium currents in different species, with a single channel conductance of approximately 100 pS ( 9 , 32 , 58 ). These results suggest a clear role for TREK-1 in the repolarization phase of the cardiac action potential ( Figure 2 ).
Figure 2 . ( A ) representative diagram of a heart showing blood flow (red line) and the different regions of interest: right atrium (RA); superior vena cava (SVC) and inferior vena cava (IVC); right ventricle (RV); pulmonary artery (PA); left atrium (LA); left ventricle (LV) and aorta (Ao). The TREK channels have been schematically represented as 1: sinoatrial node, 2: conduction system (Purkije fibres) and 3: muscle cells. ( B ) Shape of a typical action potential (top) and the conductances that generate it (bottom). Indicating the area where TREK channels are most likely to be involved (green shaded area). ( C ) Effect of TREK channel removal on intrathecal calcium [Ca 2+ ]i activity in mouse cardiomyocytes. Adapted from ( 56 , 73 ).
Finally, the variable distribution of TREK-1 in both endothelial and smooth muscle cells across different regions of the heart could facilitate precise regulation of the depolarization wave that initiates cardiac contraction ( 74 ). For example, TREK-1 is less present in myocytes of the epicardium of adult rats than in endocardial cells ( 12 ).
At the same time, TREK channels play an important role in cardiovascular diseases ( 47 ), so that its experimental withdrawal is expected to be pro-arrhythmic ( 75 ). TREK-1 has been associated with reduced right atrial channel expression in Atrial fibrillation (AF) models ( 46 ). AF is the most common cardiac arrhythmia and results from shortening of atrial effective refractory periods and from a localized deceleration of intra-atrial conduction ( 76 – 78 ). In this context, we recently have shown that verapamil (a class IV antiarrhythmic drug used in pathological conditions such as chronic angina pectoris, cardiac arrhythmias or hypertension) reduces the TREK-1 activity ( 79 ). TREK-1 channels may have a role in other pathophysiological situations, during ischemia, when purinergic agonists such as ATP cause the release of arachidonic acid (AA) ( 80 ), which lowers intracellular pH, the change of pH/AA can be detected by TREK-1 ( 63 ) and could contribute to electrophysiological disturbances in the cardiac mechano-electric feedback ( 81 ). TREK-1, plays a protective role against ischaemia-induced neuronal damage and has been shown to play a critical role in cardiac injury and during remodelling after myocardial infarction. Moreover, in TREK-1 KO animals, TREK-1 increases infarct size induced in experimental models, leading to greater systolic dysfunction than its wild-type counterpart, so that activation of TREK-1 may be an effective strategy to provide cardioprotection against ischaemia-induced damage. In addition, a study on the role of TREK-1 in the control of cardiac excitability found that TREK-1 is essential for normal sinoatrial node cell excitability and serves as a potential target for selectively regulating sinoatrial node cell function ( 56 , 57 ).
Also at peripheral level, the expression pattern of TREK channels in the vascular system has been widely demonstrated. For example, TREK-1, TREK-2 and TRAAK have been detected in various vascular structures such as the pulmonary and femoral artery and the cerebral arteries in both murids and humans, suggesting a putative role for these channels in the vascular system, particularly for TREK-1 ( 81 ). Through WB and RT-PCR, TREK mRNA was detected in rat mesenteric and pulmonary arteries ( 62 ) and TREK-1 has been suggested to influence mechanically induced endothelial signalling by modulating nitric oxide production ( 69 ). In heterologous systems it has been shown that the presence of treprostinil (a tricyclic benzidine analogue of PGI 2 used for treatment of pulmonary arterial hypertension) was able to inhibit TREK-1 and TREK-2, supporting the idea that TREK-1 could contribute to the cardiac mechano-electric feedback with a hyperpolarizing current in response to mechanical forces in the vascular system.
Concluding remarks and perspectives
Two types of currents activated by mechanical stimulation operate in the heart, on the one hand a depolarizing non-selective cationic current and on the other hand a hyperpolarizing outward current mainly transported by potassium. Despite originating some controversy ( 20 ), the family of depolarizing non-selective cationic current is mainly composed of TRP and Piezo channels ( 19 , 20 ), and responds with a wide depolarizing current that occurs mainly in the sarcolemma. Stretch-activated potassium currents are primarily driven by TREK channels, which play an important role in cardiac mechano-electrical feedback, both at the cellular level (e.g., presence in principal cells such as pacemakers and cardiomyocytes) and at the system level through their involvement in the regulation of heartbeat force and rate ( 6 , 19 , 82 ). Overall, there is now some evidence for the ability of TREK channels to control the electrical activity of the heart through central mechano-electrical feedback ( 19 ).. It has been proposed that the main function of TREK-1 is to counteract the depolarising effect induced by currents activated by mechanical stimuli, thus contributing to central mechano-electric feedback in the cardiovascular system ( 55 , 69 ) and controlling, at least in part, the early repolarisation phase and action potential transfer through the ventricular conduction system. Finally, as mentioned above, it appears that TREK channels, especially TREK-1, may play a role in nodal pacemaker activity.
The strong presence of TREK-1 could also indicate a possible role in the mechanical control of the electrical activity of the vascular periphery. However, other players must be considered in the mechano-electric feedback process. Recent work has investigated the role of Piezo 1 channels in the development of cardiac hypertrophy, showing how activation of calcium/calpain signalling through the Piezo 1 pathway contributes to the development of cardiac hypertrophy in murine models. Furthermore, Piezo 1 is a cardiac mechano-sensor that is activated in response to cardiac overload in adult animals, which in turn initiates the myocardial hypertrophic response. On the other hand, it has also been shown that Piezo 1 activation in response to mechanical stimuli triggers chemical signals that contribute to the physiological response of the heart to mechanical stress ( 83 – 85 ). These findings undoubtedly support the relevant role that Piezo channels may play in both mechano-electric feedback and cardiac pathophysiology.
In summary, TREK channels are involved in the regulation of mechanical forces both centrally and peripherally in the cardiovascular system. It should be noted that there may be other currents at play that could contribute to or even counteract TREK activity. More important, recent data have shown that antiarrhythmic drugs can interact with mechanically-gated TREK channels. There is enough evidence supporting the hypothesis that potassium outward currents driven by TREK channels play an important role not only in the normal functioning of the cardiovascular system, where its mechanical sensitivity plays a central aspect, but also in some relevant pathologies such as AF and other cardiac conditions. The expression of TREK-1 channels in the ventricle exhibits regional heterogeneity, similar to that observed in mechano-electrical feedback under physiological conditions. Consequently, this regional variability in TREK-1 channel expression has the potential to modulate mechano-electrical feedback, resulting in altered repolarization of the action potential and consequent arrhythmogenic effects ( 86 ). Although in this review we have focused on the possible role of TREK channels in cardiac mechano-electric feedback as well as their possible role in the phytopathology of the heart, there is no doubt that other MS ion channels such as Piezo channels must be taken into account in the explanation of the molecular mechanism underlying cardiac mechano-electric feedback.
Presently, a significant limitation exists in the investigation of the possible role of TREK channels in the mechano-electric feedback process due to the lack of identified specific TREK channel blockers. Nonetheless, a considerable body of evidence supports the proposition that these channels are unequivocally responsible for potassium current in response to mechanical stimuli, and given their abundant expression in the cardiovascular system, it is highly probable that they are fundamental in the feedback process. Furthermore, as previously remarked, there is clear evidence indicating that TREK channels have a relevant role in cardiac pathophysiology. However, despite the extensive evidence of TREK channel presence in various regions of the cardiovascular system, including sympathetic innervation, it is presently unknown if these channels are also present in the intracardiac ganglion, which is responsible for parasympathetic control of cardiac activity. Moreover, as the pharmacology of TREK channel usage progresses, it is conceivable that more appropriate experimental designs can be employed to elucidate the relationship between TREK channels and mechano-electric feedback more clearly.
Author contributions
Conceptualization, SH-P and JAL; validation, JAL; investigation, SH-P and JAL; resources, JAL; writing of original draft, SH-P and JAL; review and editing of manuscript, SH-P and JAL; supervision, JAL; funding acquisition, JAL All authors have read and agreed to the published version of the manuscript.
This research was funded by the Spanish government M.I.C.I.U. PID2019-109425GB-I00. All the funding was awarded to J. Antonio Lamas.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet . (2002) 36(1):411–53. doi: 10.1146/annurev.genet.36.061802.101708
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature . (2012) 483(7388):176–81. doi: 10.1038/nature10812
3. Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 channels are inherently mechanosensitive. Cell Rep . (2016) 17(7):1739–46. doi: 10.1016/j.celrep.2016.10.033
4. Douguet D, Patel A, Xu A, Vanhoutte PM, Honoré E. Piezo Ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci . (2019) 40(12):956–70. doi: 10.1016/j.tips.2019.10.002
5. Ridone P, Vassalli M, Martinac B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev . (2019) 11(5):795–805. doi: 10.1007/s12551-019-00584-5
6. Cox CD, Bavi N, Martinac B. Biophysical principles of Ion-channel-mediated mechanosensory transduction. Cell Rep . (2019) 29(1):1–12. doi: 10.1016/j.celrep.2019.08.075
7. Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K + channel. EMBO J . (1998) 17(15):4283–90. doi: 10.1093/emboj/17.15.4283
8. Bers DM. Cardiac excitation–contraction coupling. Nature . (2002) 415(6868):198–205. doi: 10.1038/415198a
9. Decher N, Kiper AK, Rinne S. Stretch-activated potassium currents in the heart: focus on TREK-1 and arrhythmias. Prog Biophys Mol Biol . (2017) 130(Pt B):223–32. doi: 10.1016/j.pbiomolbio.2017.05.005
10. Beech DJ, Kalli AC. Force sensing by piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol . (2019) 39(11):2228–39. doi: 10.1161/ATVBAHA.119.313348
11. Schmidt C, Peyronnet R. Voltage-gated and stretch-activated potassium channels in the human heart: pathophysiological and clinical significance. Herzschr Elektrophys . (2018) 29(1):36–42. doi: 10.1007/s00399-017-0541-z
CrossRef Full Text | Google Scholar
12. Tan JH, Liu W, Saint DA. Differential expression of the mechanosensitive potassium channel TREK-1 in epicardial and endocardial myocytes in rat ventricle. Exp Physiol . (2004) 89(3):237–42. doi: 10.1113/expphysiol.2003.027052
13. Fang X-Z, Zhou T, Xu J-Q, Wang Y-X, Sun M-M, He Y-J, et al. Structure, kinetic properties and biological function of mechanosensitive piezo channels. Cell Biosci . (2021) 11(1):1–20. doi: 10.1186/s13578-020-00515-y
14. Arts T, Lumens J, Kroon W, Delhaas T. Control of whole heart geometry by intramyocardial mechano-feedback: a model study. PLoS Comput Biol . (2012) 8(2):e1002369. doi: 10.1371/journal.pcbi.1002369
15. Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther . (2009) 123(3):371–85. doi: 10.1016/j.pharmthera.2009.05.009
16. Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci . (2004) 24(28):6410–5. doi: 10.1523/JNEUROSCI.1421-04.2004
17. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA . (2006) 103(44):16586–91. doi: 10.1073/pnas.0606894103
18. Canales J, Morales D, Blanco C, Rivas J, Diaz N, Angelopoulos I, Cerda O. A TR(i)P to cell migration: new roles of TRP channels in mechanotransduction and cancer. Front Physiol . (2019) 10:757. doi: 10.3389/fphys.2019.00757
19. Kohl P. Cardiac stretch-activated channels and mechano-electric coupling. In: Zipes DP, Stevenson WG, editors. Cardiac electrophysiology: From cell to bedside (seventh edition) . Elsevier (2018). p. 128–39.
20. Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Archiv—European Journal of Physiology . (2008) 455(6):1097–103. doi: 10.1007/s00424-007-0359-3
21. Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vasquez V, et al. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci . (2019) 132(23.31722978
PubMed Abstract | Google Scholar
22. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science . (2010) 330(6000):55–60. doi: 10.1126/science.1193270
23. Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science . (2018) 362(6413):464–7. doi: 10.1126/science.aau6324
24. Ranade SS, Qiu Z, Woo S-H, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA . (2014) 111(28):10347–52. doi: 10.1073/pnas.1409233111
25. Lamas JA. Mechanosensitive K2P channels, TREKking through the autonomic nervous system. In: Kamkin A, Lozinsky I, editors. Mechanically gated channels and their regulation . Dordrecht: Springer Science+Business Media (2012). p. 35–68.
26. Herrera-Pérez S, Campos-Ríos A, Rueda-Ruzafa L, Lamas JA. Contribution of K2P potassium channels to cardiac physiology and pathophysiology. Int J Mol Sci . (2021) 22(12). doi: 10.3390/ijms22126635
27. Prager-Khoutorsky M, Khoutorsky A, Bourque CW. Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron . (2014) 83(4):866–78. doi: 10.1016/j.neuron.2014.07.023
28. Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K + channels. Proc Natl Acad Sci U S A . (2014) 111(9):3614–9. doi: 10.1073/pnas.1320768111
29. Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem . (2000) 275:17412–9.
Google Scholar
30. Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci USA. (2006) 103:6859–64.
31. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem . (1999) 274:26691–6.
32. Schewe M, Sun H, Mert U, Mackenzie A, Pike, ACW, Schulz F et al. A pharmacological master key mechanism that unlocks the selectivity filter gate in K(+) channels. Science (2019) 363:875–80.
33. Niemeyer MI, Cid LP, Gonzalez W, Sepulveda FV. Gating, regulation, and structure in K2P K+ channels: In varietate Concordia? Mol Pharmacol . (2016) 90:309–17.27268784
34. Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem . (2008) 283(28):19448–55. doi: 10.1074/jbc.M801273200
35. Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol . (2010) 588(Pt 17):3149–56. doi: 10.1113/jphysiol.2010.192344
36. Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, et al. K2p channel gating mechanisms revealed by structures of TREK-2 and a complex with prozac. Science . (2015) 347(6227):1256–9. doi: 10.1126/science.1261512
37. Loussouarn G, Rose T, Nichols CG. Structural basis of inward rectifying potassium channel gating. Trends Cardiovasc Med . (2002) 12(6):253–8. doi: 10.1016/S1050-1738(02)00170-6
38. Yellen G. The voltage-gated potassium channels and their relatives. Nature . (2002) 419(6902):35–42. doi: 10.1038/nature00978
39. Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Andres-Enguix I, et al. The pore structure and gating mechanism of K2P channels. EMBO J . (2011) 30(17):3607–19. doi: 10.1038/emboj.2011.268
40. Bagriantsev SN, Clark KA, Minor DL Jr. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J . (2012) 31(15):3297–308. doi: 10.1038/emboj.2012.171
41. Kopec W, Rothberg BS, de Groot BL. Molecular mechanism of a potassium channel gating through activation gate-selectivity filter coupling. Nat Commun . (2019) 10(1):5366. doi: 10.1038/s41467-019-13227-w
42. Renigunta V, Schlichthorl G, Daut J. Much more than a leak: structure and function of K(2)p-channels. Pflugers Arch . (2015) 467(5):867–94. doi: 10.1007/s00424-015-1703-7
43. Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J . (2005) 24(1):44–53. doi: 10.1038/sj.emboj.7600494
44. Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K + channel. Nature . (2014) 516(7529):126–30. doi: 10.1038/nature14013
45. Wiedmann F, Rinne S, Donner B, Decher N, Katus HA, Schmidt C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog Biophys Mol Biol . (2021) 159:126–35.32553901
46. Schmidt C, Wiedmann F, Tristram F, Anand P, Wenzel W, Lugenbiel P, et al. Cardiac expression and atrial fibrillation-associated remodeling of K(2)p2.1 (TREK-1) K(+) channels in a porcine model. Life Sci . (2014) 97(2):107–15. doi: 10.1016/j.lfs.2013.12.006
47. Brennecke JT, de Groot BL. Mechanism of mechanosensitive gating of the TREK-2 potassium channel. Biophys J . (2018) 114(6):1336–43. doi: 10.1016/j.bpj.2018.01.030
48. Lolicato M, Riegelhaupt PM, Arrigoni C, Clark KA, Minor DL Jr. Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K2P channels. Neuron . (2014) 84(6):1198–212. doi: 10.1016/j.neuron.2014.11.017
49. Peyronnet R, Sharif-Naeini R, Folgering JH, Arhatte M, Jodar M, El Boustany C, et al. Mechanoprotection by polycystins against apoptosis is mediated through the opening of stretch-activated K(2P) channels. Cell Rep . (2012) 1(3):241–50. doi: 10.1016/j.celrep.2012.01.006
50. Poole K, Moroni M, Lewin GR. Sensory mechanotransduction at membrane-matrix interfaces. Pflugers Arch . (2015) 467(1):121–32. doi: 10.1007/s00424-014-1563-6
51. Mikhailov N, Leskinen J, Fagerlund I, Poguzhelskaya E, Giniatullina R, Gafurov O, et al. Mechanosensitive meningeal nociception via piezo channels: implications for pulsatile pain in migraine? Neuropharmacology . (2019) 149:113–23. doi: 10.1016/j.neuropharm.2019.02.015
52. Roh J, Hwang SM, Lee SH, Lee K, Kim YH, Park CK, et al. Functional expression of Piezo1 in dorsal root ganglion (DRG) neurons. Int J Mol Sci . (2020) 21(11).
53. Liu W, Saint DA. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin Exp Pharmacol Physiol . (2004) 31(3):174–8. doi: 10.1111/j.1440-1681.2004.03964.x
54. Abraham DM, Lee TE, Watson LJ, Mao L, Chandok G, Wang HG, et al. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J Clin Invest . (2018) 128(11):4843–55. doi: 10.1172/JCI95945
55. Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, et al. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res . (2006) 69(1):86–97. 16248991
56. Kamatham S, Waters CM, Schwingshackl A, Mancarella S. TREK-1 protects the heart against ischemia-reperfusion-induced injury and from adverse remodeling after myocardial infarction. Pflugers Arch . (2019) 471(10):1263–72. doi: 10.1007/s00424-019-02306-y
57. Urthi SD, Wu X, Qian L, Amari F, Onal B, Li N, et al. Two-Pore K+ channel TREK-1 regulates sinoatrial node membrane excitability. J Am Heart Assoc . (2016) 5(4):e002865. doi: 10.1161/JAHA.115.002865
58. Bodnar M, Schlichthorl G, Daut J. The potassium current carried by TREK-1 channels in rat cardiac ventricular muscle. Pflugers Arch . (2015) 467(5):1069–79. doi: 10.1007/s00424-014-1678-9
59. Schmidt C, et al. Upregulation of K(2P)3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation . (2015) 132(2):82–92. doi: 10.1161/CIRCULATIONAHA.114.012657
60. Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ Res . (2001) 89(4):336–42. doi: 10.1161/hh1601.094979
61. Lesage F, Terrenoire C, Romey G, Lazdunski M. An increased TREK-1-like potassium current in ventricular myocytes during rat cardiac hypertrophy. J Cardiovasc Pharm . (2013) 61(4):302–10. doi: 10.1097/FJC.0b013e318280c5a9
62. Meadows HJ, Chapman CG, Duckworth DM, Kelsell RE, Murdock PR, Nasir S, et al. Functional evidence of a role for two - pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol . (2004) 142(1):192–202. doi: 10.1038/sj.bjp.0705691
63. Gu W, Schlichthorl G, Hirsch JR, Engels H, Karschin C, Karschin A, et al. Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+ current ITREK in cardiomyocytes. J Biol Chem . (2000) 275(50):39110–6. doi: 10.1074/jbc.M008192200
64. Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, et al. A neuronal two P domain K + channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J . (1998) 17(12):3297–308. doi: 10.1093/emboj/17.12.3297
65. Lesage F, Maingret F, Lazdunski M. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive K(+) channel. FEBS Lett . (2000) 471(2-3):137–40. doi: 10.1016/S0014-5793(00)01388-0
66. Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K + channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and gs, gi, and gq protein-coupled receptors. J Biol Chem . (2000) 275(37):28398–405. doi: 10.1074/jbc.M002822200
67. Meadows HJ, Chapman CG, Duckworth DM, Kelsell RE, Murdock PR, Nasir S, et al. The neuroprotective agent sipatrigine (BW619C89) potently inhibits the human tandem pore-domain K(+) channels TREK-1 and TRAAK. Brain Res . (2001) 892(1):94–101. doi: 10.1016/S0006-8993(00)03239-X
68. Gu W, Schlichthorl G, Hirsch JR, Engels H, Karschin C, Karschin A, et al. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol . (2002) 539(Pt 3):657–68. doi: 10.1113/jphysiol.2001.013432
69. Goonetilleke L, Quayle J. TREK-1 K(+) channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc Ther . (2012) 30(1):e23–9. doi: 10.1111/j.1755-5922.2010.00227.x
70. Froese A, Breher SS, Waldeyer C, Schindler RF, Nikolaev VO, Rinne S. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. J Clin Invest . (2012) 122(3):1119–30. doi: 10.1172/JCI59410
71. Hund TJ, Snyder JS, Wu X, Glynn P, Koval OM, Onal B, et al. β IV-spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc Res . (2014) 102(1):166–75. doi: 10.1093/cvr/cvu008
72. Reed A, Kohl P, Peyronnet R. Molecular candidates for cardiac stretch-activated ion channels. Glob Cardiol Sci Pract . (2014) 2014(2):9–25.25405172
73. Klabunde R. 2—electrical Activity of the heart, in Cardiovascular physiology concepts . (2011), Lippincott Williams & Wilkins. p. 9–40.
74. Enyedi P, Czirjak G. Molecular background of leak K + currents: two-pore domain potassium channels. Physiol Rev . (2010) 90(2):559–605. doi: 10.1152/physrev.00029.2009
75. Decher N, Ortiz-Bonnin B, Friedrich C, Schewe M, Kiper AK, Rinne S, et al. Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol Med . (2017) 9(4):403–14. doi: 10.15252/emmm.201606690
76. Ravens U, Odening KE. Atrial fibrillation: therapeutic potential of atrial K(+) channel blockers. Pharmacol Ther . (2017) 176:13–21. doi: 10.1016/j.pharmthera.2016.10.003
77. Limberg SH, Netter MF, Rolfes C, Rinne S, Schlichthorl G, Zuzarte M, et al. TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cell Physiol Biochem . (2011) 28(4):613–24. doi: 10.1159/000335757
78. Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res . (2005) 96(9):1022–9. doi: 10.1161/01.RES.0000165480.82737.33
79. Herrera-Perez S, Rueda-Ruzafa L, Campos-Rios A, Fernandez-Fernandez D, Lamas JA. Antiarrhythmic calcium channel blocker verapamil inhibits trek currents in sympathetic neurons. Front Pharmacol . (2022) 13:997188. doi: 10.3389/fphar.2022.997188
80. Basavarajappa BS, Yalamanchili R, Cooper TB, Hungund BL. The endocannabinoid system. In: Vizi ES, Lajtha A, editors. Handbook of neurochemistry and molecular neurobiology: neurotransmitter systems . Springer Science & Business Media (2008). p. 343–83.
81. Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J . (2009) 38(3):305–18. doi: 10.1007/s00249-008-0326-8
82. Sigurdson WJ, Morris CE. Stretch-activated ion channels in growth cones of snail neurons. J Neurosci . (1989) 9(8):2801–8. doi: 10.1523/JNEUROSCI.09-08-02801.1989
83. Zhang Y, Su SA, Li W, Ma Y, Shen J, Wang Y, et al. Piezo1-Mediated mechanotransduction promotes cardiac hypertrophy by impairing calcium homeostasis to activate calpain/calcineurin signaling. Hypertension . (2021) 78(3):647–60. doi: 10.1161/HYPERTENSIONAHA.121.17177
84. Yu Z-Y, Gong H, Kesteven S, Guo Y, Wu J, Li JV, et al. Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nature Cardiovascular Research . (2022) 1(6):577–91. doi: 10.1038/s44161-022-00082-0
85. Jiang F, Yin K, Wu K, Zhang M, Wang S, Cheng H, et al. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun . (2021) 12(1):869. doi: 10.1038/s41467-021-21178-4
86. Bechard E, Bride J, Le Guennec JY, Brette F, Demion M. TREK-1 in the heart: potential physiological and pathophysiological roles. Front Physiol . (2022) 13:1095102. doi: 10.3389/fphys.2022.1095102
Keywords: TREK, mechanobiology, cardiovascular system, heart, mechano-feedback
Citation: Herrera-Pérez S and Lamas JA (2023) TREK channels in Mechanotransduction: a Focus on the Cardiovascular System. Front. Cardiovasc. Med. 10:1180242. doi: 10.3389/fcvm.2023.1180242
Received: 6 March 2023; Accepted: 26 April 2023; Published: 23 May 2023.
Reviewed by:
© 2023 Herrera-Pérez and Lamas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salvador Herrera-Pérez [email protected] José Antonio Lamas [email protected]
This article is part of the Research Topic
New Discoveries on Calcium Handling in Cardiovascular Pathology
- USA office +1 (323) 644-4063
TV broadcasting in the trains of the Moscow Metro
- News & Events
- Press release
Tomsk, Russia, January 23, 2020. The Elecard company took part in the large-scale project on content preparation and broadcasting of 12 channels in the Moscow underground trains.
The Moscow Metro is the backbone of the Moscow transport system. It consists of 15 lines and 269 stations, on which more than 12 thousand trains are passed daily. There are 6200 video screens installed in the subway cars, which broadcast TV channels, information videos, and commercials. The monitors are designed for a large audience: more than 7 million people use the metro every day.
Elecard, SoftLab-NSK, and Stream Labs developed and implemented the hardware and software complex for preparation, broadcasting, and monitoring of TV channels in metro trains of 12 lines. This solution is based on the transcoder Elecard CodecWorks and high-density servers. CodecWorks supports H.265/HEVC, which is especially important in this project, as the communication bandwidth in the rolling stock is limited. HEVC allows broadcasting video of higher quality due to a higher degree of compression, as opposed to the AVC format. The solution guarantees stable broadcasting, as it includes redundancy of all system components. If an error occurs, the schema will automatically switch to the reserve source, and viewers will not notice a failure.
The Stream Labs provided the solution for the head-end station monitoring. The technologies of the SoftLab-NSK were used to implement the playout system. The solution supports targeted advertising adjusted for the content availability and duration. A playlist with a specific set of informational videos and commercials is prepared for each subway line. The system is integrated with external systems of advertising content delivery.
“ Metropolitan is a unique facility that has its own specifics in terms of standards and operation. It is important to understand and consider this in our work. We provided exclusive technical support during testing and implementation of the solution ," said Nikolay Milovanov, Elecard CEO.
" We came up with the task to implement broadcasting of media content with targeted advertising in Moscow Metro. We turned to Elecard with request to provide a high-quality software solution. The implemented solution allows us to broadcast not only commercials but also sports ans cultural events. On behalf of the company I would like to express my gratitude to Elecard employees for the project implementation and professional support at all stages of the system integration ," comments Maxim Shemegon, Development Director of the Moscow Metro State Unitary Enterprise.
Elecard CodecWorks is a professional software solution for real-time decoding, encoding, and transcoding into MPEG-2/AVC/HEVC with up to 16K resolution supporting multi-screen encoding and HLS/MPEG-DASH adaptive streaming technologies. CodecWorks has passed through comprehensive testing and guarantees high performance and continuous content delivery suitable for projects of any scale and complexity.
About Elecard Elecard provides software products for encoding, decoding, processing, receiving, and transmission of video and audio data in different formats (H.265/HEVC, H.264/AVC, MPEG-4, MPEG-2). Elecard is based in the United States, Canada, Russia, and Vietnam. For more information, please visit www.elecard.com. The company offers a wide range of reference designs for professional digital TV broadcasting market, which includes streaming, transcoding, video-on-demand servers, professional software products, and software development kits. Elecard is based in the United States, Russia, and Vietnam. For more information, please visit www.elecard.com.
Contacts: Tel.: +7 (3822) 488-585 [email protected]
About the Moscow Metro The Moscow Metro State Unitary Enterprise is an organization that provides rail transportation services for passengers in Moscow. It includes the Moscow Metro and the monorail. The Moscow Metro is the basis of the Moscow transport system, which consists of 15 lines and 269 stations.
Contacts: Tel.: +7 (495) 539-54-54
On the topic
Latest news.
Elecard ViCont is a unique system for delivering targeted content to viewers
Elecard team welcomes you to try our updated version of CodecWorks offering the possibility to encrypt TS and OTT streams and provide reliable content protection
Stay connected

Turn Your Curiosity Into Discovery
Latest facts.

The Best AI Photo Editor of 2024 A Comprehensive Review

6 Facts You Didnt Know About Ecommerce Call Center Outsourcing
40 facts about elektrostal.
Written by Lanette Mayes
Modified & Updated: 02 Mar 2024
Reviewed by Jessica Corbett

Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to captivate you.
This article will provide you with 40 fascinating facts about Elektrostal, giving you a better understanding of why this city is worth exploring. From its origins as an industrial hub to its modern-day charm, we will delve into the various aspects that make Elektrostal a unique and must-visit destination.
So, join us as we uncover the hidden treasures of Elektrostal and discover what makes this city a true gem in the heart of Russia.
Key Takeaways:
- Elektrostal, known as the “Motor City of Russia,” is a vibrant and growing city with a rich industrial history, offering diverse cultural experiences and a strong commitment to environmental sustainability.
- With its convenient location near Moscow, Elektrostal provides a picturesque landscape, vibrant nightlife, and a range of recreational activities, making it an ideal destination for residents and visitors alike.
Known as the “Motor City of Russia.”
Elektrostal, a city located in the Moscow Oblast region of Russia, earned the nickname “Motor City” due to its significant involvement in the automotive industry.
Home to the Elektrostal Metallurgical Plant.
Elektrostal is renowned for its metallurgical plant, which has been producing high-quality steel and alloys since its establishment in 1916.
Boasts a rich industrial heritage.
Elektrostal has a long history of industrial development, contributing to the growth and progress of the region.
Founded in 1916.
The city of Elektrostal was founded in 1916 as a result of the construction of the Elektrostal Metallurgical Plant.
Located approximately 50 kilometers east of Moscow.
Elektrostal is situated in close proximity to the Russian capital, making it easily accessible for both residents and visitors.
Known for its vibrant cultural scene.
Elektrostal is home to several cultural institutions, including museums, theaters, and art galleries that showcase the city’s rich artistic heritage.
A popular destination for nature lovers.
Surrounded by picturesque landscapes and forests, Elektrostal offers ample opportunities for outdoor activities such as hiking, camping, and birdwatching.
Hosts the annual Elektrostal City Day celebrations.
Every year, Elektrostal organizes festive events and activities to celebrate its founding, bringing together residents and visitors in a spirit of unity and joy.
Has a population of approximately 160,000 people.
Elektrostal is home to a diverse and vibrant community of around 160,000 residents, contributing to its dynamic atmosphere.
Boasts excellent education facilities.
The city is known for its well-established educational institutions, providing quality education to students of all ages.
A center for scientific research and innovation.
Elektrostal serves as an important hub for scientific research, particularly in the fields of metallurgy, materials science, and engineering.
Surrounded by picturesque lakes.
The city is blessed with numerous beautiful lakes, offering scenic views and recreational opportunities for locals and visitors alike.
Well-connected transportation system.
Elektrostal benefits from an efficient transportation network, including highways, railways, and public transportation options, ensuring convenient travel within and beyond the city.
Famous for its traditional Russian cuisine.
Food enthusiasts can indulge in authentic Russian dishes at numerous restaurants and cafes scattered throughout Elektrostal.
Home to notable architectural landmarks.
Elektrostal boasts impressive architecture, including the Church of the Transfiguration of the Lord and the Elektrostal Palace of Culture.
Offers a wide range of recreational facilities.
Residents and visitors can enjoy various recreational activities, such as sports complexes, swimming pools, and fitness centers, enhancing the overall quality of life.
Provides a high standard of healthcare.
Elektrostal is equipped with modern medical facilities, ensuring residents have access to quality healthcare services.
Home to the Elektrostal History Museum.
The Elektrostal History Museum showcases the city’s fascinating past through exhibitions and displays.
A hub for sports enthusiasts.
Elektrostal is passionate about sports, with numerous stadiums, arenas, and sports clubs offering opportunities for athletes and spectators.
Celebrates diverse cultural festivals.
Throughout the year, Elektrostal hosts a variety of cultural festivals, celebrating different ethnicities, traditions, and art forms.
Electric power played a significant role in its early development.
Elektrostal owes its name and initial growth to the establishment of electric power stations and the utilization of electricity in the industrial sector.
Boasts a thriving economy.
The city’s strong industrial base, coupled with its strategic location near Moscow, has contributed to Elektrostal’s prosperous economic status.
Houses the Elektrostal Drama Theater.
The Elektrostal Drama Theater is a cultural centerpiece, attracting theater enthusiasts from far and wide.
Popular destination for winter sports.
Elektrostal’s proximity to ski resorts and winter sport facilities makes it a favorite destination for skiing, snowboarding, and other winter activities.
Promotes environmental sustainability.
Elektrostal prioritizes environmental protection and sustainability, implementing initiatives to reduce pollution and preserve natural resources.
Home to renowned educational institutions.
Elektrostal is known for its prestigious schools and universities, offering a wide range of academic programs to students.
Committed to cultural preservation.
The city values its cultural heritage and takes active steps to preserve and promote traditional customs, crafts, and arts.
Hosts an annual International Film Festival.
The Elektrostal International Film Festival attracts filmmakers and cinema enthusiasts from around the world, showcasing a diverse range of films.
Encourages entrepreneurship and innovation.
Elektrostal supports aspiring entrepreneurs and fosters a culture of innovation, providing opportunities for startups and business development.
Offers a range of housing options.
Elektrostal provides diverse housing options, including apartments, houses, and residential complexes, catering to different lifestyles and budgets.
Home to notable sports teams.
Elektrostal is proud of its sports legacy, with several successful sports teams competing at regional and national levels.
Boasts a vibrant nightlife scene.
Residents and visitors can enjoy a lively nightlife in Elektrostal, with numerous bars, clubs, and entertainment venues.
Promotes cultural exchange and international relations.
Elektrostal actively engages in international partnerships, cultural exchanges, and diplomatic collaborations to foster global connections.
Surrounded by beautiful nature reserves.
Nearby nature reserves, such as the Barybino Forest and Luchinskoye Lake, offer opportunities for nature enthusiasts to explore and appreciate the region’s biodiversity.
Commemorates historical events.
The city pays tribute to significant historical events through memorials, monuments, and exhibitions, ensuring the preservation of collective memory.
Promotes sports and youth development.
Elektrostal invests in sports infrastructure and programs to encourage youth participation, health, and physical fitness.
Hosts annual cultural and artistic festivals.
Throughout the year, Elektrostal celebrates its cultural diversity through festivals dedicated to music, dance, art, and theater.
Provides a picturesque landscape for photography enthusiasts.
The city’s scenic beauty, architectural landmarks, and natural surroundings make it a paradise for photographers.
Connects to Moscow via a direct train line.
The convenient train connection between Elektrostal and Moscow makes commuting between the two cities effortless.
A city with a bright future.
Elektrostal continues to grow and develop, aiming to become a model city in terms of infrastructure, sustainability, and quality of life for its residents.
In conclusion, Elektrostal is a fascinating city with a rich history and a vibrant present. From its origins as a center of steel production to its modern-day status as a hub for education and industry, Elektrostal has plenty to offer both residents and visitors. With its beautiful parks, cultural attractions, and proximity to Moscow, there is no shortage of things to see and do in this dynamic city. Whether you’re interested in exploring its historical landmarks, enjoying outdoor activities, or immersing yourself in the local culture, Elektrostal has something for everyone. So, next time you find yourself in the Moscow region, don’t miss the opportunity to discover the hidden gems of Elektrostal.
Q: What is the population of Elektrostal?
A: As of the latest data, the population of Elektrostal is approximately XXXX.
Q: How far is Elektrostal from Moscow?
A: Elektrostal is located approximately XX kilometers away from Moscow.
Q: Are there any famous landmarks in Elektrostal?
A: Yes, Elektrostal is home to several notable landmarks, including XXXX and XXXX.
Q: What industries are prominent in Elektrostal?
A: Elektrostal is known for its steel production industry and is also a center for engineering and manufacturing.
Q: Are there any universities or educational institutions in Elektrostal?
A: Yes, Elektrostal is home to XXXX University and several other educational institutions.
Q: What are some popular outdoor activities in Elektrostal?
A: Elektrostal offers several outdoor activities, such as hiking, cycling, and picnicking in its beautiful parks.
Q: Is Elektrostal well-connected in terms of transportation?
A: Yes, Elektrostal has good transportation links, including trains and buses, making it easily accessible from nearby cities.
Q: Are there any annual events or festivals in Elektrostal?
A: Yes, Elektrostal hosts various events and festivals throughout the year, including XXXX and XXXX.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
Share this Fact:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pharmacol
Role of TREK-1 in Health and Disease, Focus on the Central Nervous System
TREK-1 is the most studied background K 2P channel. Its main role is to control cell excitability and maintain the membrane potential below the threshold of depolarization. TREK-1 is multi-regulated by a variety of physical and chemical stimuli which makes it a very promising and challenging target in the treatment of several pathologies. It is mainly expressed in the brain but also in heart, smooth muscle cells, endocrine pancreas, and prostate. In the nervous system, TREK-1 is involved in many physiological and pathological processes such as depression, neuroprotection, pain, and anesthesia. These properties explain why many laboratories and pharmaceutical companies have been focusing their research on screening and developing highly efficient modulators of TREK-1 channels. In this review, we summarize the different roles of TREK-1 that have been investigated so far in attempt to characterize pharmacological tools and new molecules to modulate cellular functions controlled by TREK-1.
Introduction
In the brain, ion channels regulate a variety of cellular processes such as neurotransmitter release, neuronal excitability, and plasticity. They are crucial in the generation and propagation of action potentials. Dysfunction of these channels causes several pathologies called channelopathies. In order to treat these channelopathies, many ion channels have been targets of small molecules and biological drugs. Overall, ion channels represent 19% of all human protein targets ( Santos et al., 2017 ). To date, 177 ion channels-based drugs have been approved as treatments for various pathologies and disorders ( Santos et al., 2017 ). Central nervous system (CNS) pathologies were associated with dysregulation of ion channels such as voltage gated Na + , K + , and Ca 2+ channels. Calcium channels are the most studied among ion channels since Ca 2+ ions in addition to controlling the cell excitability, they act as second messengers that convert electrical signals into chemical activity ( Pietrobon, 2002 ). On the other hand, to control neuronal excitability, potassium channels repolarize neurons by extruding K + ions to the extracellular space. Neurological potassium channelopathies are mainly caused by dysregulation of voltage-dependent potassium channels ( Benatar, 2000 ) and also of the inwardly rectifying potassium channels (Kir) which provokes an hyperexcitability seen in epilepsy ( Spillane et al., 2016 ). Mutations in the KCNT1 gene encoding for sodium -activated potassium channels cause a rare infantile encephalopathy called the migrating partial seizures of infancy ( Barcia et al., 2012 ). In other cases, mutations of KCNQ2 encoding for Kv7.2 channel provoke severe epileptic encephalopathies ( Weckhuysen et al., 2012 ). The inward-rectifier potassium channel Kir 6.2 is encoded by KCNJ11 gene forms the major subunit of the ATP-sensitive potassium channel. Mutations in this gene cause DEND syndrome, a very rare severe form of neonatal diabetes mellitus characterized by developmental delay, epilepsy and neonatal diabetes ( Gloyn et al., 2004 ). Kv7.2 and Kv7.3 are the main components of the slow voltage-gated M-channel which regulates neuronal excitability ( Brown and Passmore, 2009 ). Loss of function mutations of the KCNQ2 and KCNQ3 genes encoding, respectively, for Kv7.2 and Kv7.3 causes benign familial neonatal convulsions ( Spillane et al., 2016 ). A dominant missense mutation in KCNMA1 gene encoding for pore-forming α-subunit of the large conductance calcium-sensitive potassium channel (BK), has been associated with a form of generalized epilepsy and paroxysmal dyskinesia ( Du et al., 2005 ).
Some mutations in K 2P channels were reported to cause several pathologies. Birk Barel mental retardation dysmorphism syndrome is caused by a missense mutation in the maternal copy of KCNK9 gene which encodes for TASK-3 (K 2P 9.1). Loss of function of the channel was observed in homodimers and also when it forms heterodimer with TASK-1 (K 2P .3.1) ( Barel et al., 2008 ). Recently, de novo a gain of function missense mutation of KCNK4 gene encoding for TRAAK channel, was reported to cause recognizable neurodevelopmental syndrome characterized by a facial dysmorphism, hypertrichosis, epilepsy, intellectual disability/developmental delay and gingival overgrowth ( Bauer et al., 2018 ). A frameshift mutation (F139Wfsx24) in KCNK18 encoding for the calcium-activated K 2P channel TRESK channel was associated with migraine with aura ( Lafreniere et al., 2010 ). Channelopathies caused by dysfunction of potassium channels are of high interest for researchers since they may present interesting targets for potential treatments.
The most recent discovered family of two-pore domain potassium channels (K 2P ) are regulated by a variety of chemical and physical stimuli ( Lesage and Lazdunski, 2000 ). In this review, we will focus on describing the role of one of the most studied K 2P channel, TREK-1 channel in health and disease. In addition to discussing the recent pharmacological modulators of its activity.
Trek-1 Channel
TREK-1, named KCNK2 or K 2P 2.1 belongs to a large family of K 2P channels containing 15 members grouped in six subfamilies. K 2P channels are the most recent class of K + channels discovered. K 2P channels or the two-pore domain potassium channels are tandems of four transmembrane segments (M1–M4) containing two-pore domain (P1 and P2) ( Figure 1 , ,2). 2 ). They possess an extended M1-P1 extracellular loop and cytosolic N- and C-termini. K 2P channels have a unique pore signature sequence Gly-Tyr(Phe)-Gly in the 1st pore (P1) and Gly-Leu(Phe)-Gly in the 2nd pore (P2) ( Honore, 2007 ; Figure 1 ). TREK-1 was first cloned from the mouse brain ( Fink et al., 1996 ; Figure 3 ). TREK-1 was named after TWIK-1 channel the first cloned K 2P channel ( Lesage et al., 1996 ). TREK-1 shares 28% sequence homology with TWIK-1 channel. TREK-1 is highly expressed in brain and lung, but is also present in kidney, heart and skeletal muscle. When we look at the brain localization, TREK-1 is highly expressed in several regions of the brain such as the olfactory bulb, the hippocampus, the cerebellum and the cortex ( Fink et al., 1996 ).

Structure and classification of K 2P channels. The family of K 2P channels is composed of 15 members grouped in six subfamilies. K 2P channels are two-pore domain potassium channels and the most recent class of K + channels discovered. They assemble as dimers of four transmembrane segments (M1–M4) and two-pore domain (P1 and P2). They have an extended M1-P1 extracellular loop and cytosolic N- and C-termini. K 2P channels have a unique pore signature sequence Gly-Tyr(Phe)-Gly in the 1st pore (P1) and Gly-Leu(Phe)-Gly in the 2nd pore (P2).

Polymodal TREK-1 regulation. TREK-1 is multiregulated by a variety of physical and chemical stimuli. TREK-1 possesses different protein partners such as AKAP150, β-COP, Mtap2, and sortilin. Sortilin interacts with TREK-1 an address it to the plasma membrane. Spadin is a synthetic peptide derived from sortilin which was shown to block TREK-1 with high affinity. Spadin antidepressant activity appears to be mediated through PI3K and Akt activation. TREK-1 is involved in numerous CNS pathologies such as depression, ischemia, epilepsy and pain.

Key milestones in discovery of TREK-1 channels. The scheme presents the major dates from the cloning of TREK-1 channels to the discovery of its role in physiology and pathology. AA, arachidonic acid; GPCR, G-protein Coupled Receptor; STAR ∗ D, Sequenced Treatment Alternatives to Relieve Depression. Green and Red triangles represent TREK-1 activation and inhibition processes, respectively.
TREK-1 displays an outward rectification in symmetrical K + condition due to an external Mg 2+ block present at negative potential and to a voltage-dependence mechanism ( Maingret et al., 2002 ). Despite the absence of a voltage-sensing domain in K 2P channels, TREK-1 and some other K 2P channels show a strong voltage-dependency. The provenance of this voltage sensitivity comes from an ion-flux gating mechanism and the movement of three to four K + ions into the high electric field of an inactive selectivity filter ( Schewe et al., 2016 ). However, this voltage-dependency is switched off by physiological stimuli such as arachidonic acid (AA) and Phosphatidylinositol bisphosphate (PIP 2 ), which convert TREK-1 to classical leak channels ( Schewe et al., 2016 ). TREK-1 with TREK-2 and TRAAK are mechano- and thermo-sensitive K 2P channels ( Honore, 2007 ). They are opened by stretch and cell swelling. TREK-1, TREK-2, and TRAAK knock-out mice are hypersensitive to mechanical force, they show mechanical allodynia and hyperalgesia during inflammation ( Brohawn, 2015 ). It was reported that the mechanical force is transmitted to TREK-1 and TRAAK directly through the lipid bilayer ( Brohawn et al., 2014 ). These channels open rapidly in response to tension and have a low threshold and broad range of tension activation ( Brohawn, 2015 ).
Physiological Regulation of TREK-1 Channels
TREK-1 channel is regulated by various physical and chemical stimuli ( Figure 2 ). TREK-1 is opened by mechanical stretch in cell-attached and inside-out configurations in both COS transfected cells and oocytes ( Patel et al., 1998 ). TREK-1 opening occurs at all positive and negative potentials. When the cytoskeleton is disrupted with colchicine or cytochalasin D, TREK-1 activation by stretch was not altered ( Patel et al., 1998 ). TREK-1 was shown to be sensitive to temperature variation in different type of cells expressing TREK-1. Heat gradually and reversibly activates TREK-1 reaching a maximum of activation at 37°C. However, at 12°C TREK-1 basal current is suppressed ( Maingret et al., 2000 ). TREK-1 heat activation needs cell integrity and cytosolic components since TREK-1 is insensitive to heat when patch membrane is excised ( Maingret et al., 2000 ). TREK-1 is activated by polyunsaturated fatty acids (PUFA) such as AA in different patch-clamp configurations: whole-cell, cell-attached, inside-out and outside-out configurations ( Patel et al., 1998 ). AA activates TREK-1 with a dose-dependent manner and requires C-terminal part of the channel which is crucial for the activation and the inhibition following phosphorylation by the protein kinase A (PKA). PIP 2 stimulates native striatal TREK-1 current in inside-out patch configuration ( Chemin et al., 2005 ). In Xenopus oocytes expressing TREK-1, PIP 2 hydrolysis inhibits TREK-1 channel by modulating its voltage dependence ( Lopes et al., 2005 ). It is also known that internal acidosis induces an activation of TREK-1 channels with the involvement of C-terminal part ( Maingret et al., 1999 ). TREK subfamily members including TREK-1, TREK-2, and TRAAK are sensitive to phosphatidic acid (PA) which results from the hydrolysis of phosphatidylcholine by phospholipase D (PLD). It was shown that TREK-1 and TREK-2 but not TRAAK are positively regulated by PLD2 which selectively binds to their C-terminal domains ( Comoglio et al., 2014 ).
TREK-1 Partner Proteins
Proteomic approach based on immunoprecipitation and mass spectrometry analysis of native channel complexes allowed the identification of a scaffolding protein called AKAP150 ( Sandoz et al., 2006 ; Figure 2 ). A-kinase anchoring protein (AKAP) anchors the regulatory subunit of PKA in proximity of substrates. AKAP150 forms signaling complexes within the neuron, these complexes are composed of PKA, PKC, PP2B (protein phosphatase 2B), PSD-95, SAP97 and ion channels ( Esseltine and Scott, 2013 ). When anchored by AKAP150 within the post-M4 region, TREK-1 becomes an active leak channel insensitive to the stimuli, internal acidification AA, or mechanical stretch ( Sandoz et al., 2006 ). The microtubule associated protein Mtap2 is another protein reported to associate with TREK-1 in the brain ( Sandoz et al., 2008 ). Mtap2 binds to microtubules and stabilizes them. TREK-1 and Mtap2 colocalize in several brain regions such as the hippocampus, cerebellum, olfactory bulb, striatum and cortex. Similarly to AKAP150, Mtap2 binding to TREK-1 increases its activity ( Sandoz et al., 2008 ). This increase in TREK-1 current amplitude is not due to a direct interaction with Mtap2 but results from an increase in the expression level of the channel at the plasma membrane. Within the neuron, TREK-1, AKAP150 and Mtap2 are simultaneously found in the post-synaptic terminals. This signaling complex regulates TREK-1 channel activity and its trafficking to the plasma membrane.
Using the yeast two-hybrid technique of the human cDNA library on the N-terminal region of TREK-1, a direct β-COP-TREK-1 interaction was reported ( Kim et al., 2010 ). β-COP is a subunit of Coat Protein Complex I (COPI) whose role is to form coated vesicles and manage the retrograde traffic from the Golgi back to the endoplasmic reticulum (ER) or between different compartments inside the Golgi ( Gomez-Navarro and Miller, 2016 ). β-COP depletion was found to decrease membrane expression of the cystic fibrosis transmembrane conductance regulator (CFTR) channel. Mutations in this chloride channel cause cystic fibrosis lung disease ( Rennolds et al., 2008 ). β-COP increases TREK-1 surface expression and current amplitude. β-COP is involved in the forward transport of TREK-1 through a direct interaction with N-terminal part of the channel ( Kim et al., 2010 ).
The most recently discovered protein partner of TREK-1 is sortilin ( Petersen et al., 1997 ; Figure 2 ), also known as neurotensin receptor-3 (NTSR 3 ) ( Mazella et al., 1998 ). Sortilin is synthetized as a precursor called prosortilin which is cleaved in the trans-Golgi compartment by the proprotein convertase furin to generate a mature sortilin and the release of a 44 amino-acid named the propeptide (PE) ( Munck Petersen et al., 1999 ). NTSR 3 is made of a large luminal domain, a single transmembrane segment and a short C-terminal moiety. The amount of NTSR 3 expressed at the plasma membrane does not exceed 10% while the majority is expressed intracellularly and involved mainly in intracellular trafficking. Both NTSR 3 and TREK-1 are markedly expressed in the prefrontal (PFC) and cingulate cortex, the amygdala, the hippocampus, the nucleus accumbens, the dorsal raphe nucleus (DRN) and the hypothalamus. The expression of TREK-1 was enhanced at the plasma membrane in COS-7 cells co-transfected with NTSR 3 ( Mazella et al., 2010 ).
TREK-1 Heterodimerization
The first K 2P member cloned TWIK-1 ( Lesage et al., 1996 ), is expressed with TREK-1 in astrocytes ( Zhou et al., 2009 ). The expression of a functional TWIK-1 channel necessitates its heterodimerization with TREK-1 to mediate the passive conductance in astrocytes ( Hwang et al., 2014 ). As cited earlier in this review, TREK subfamily includes TREK-1, TREK-2 and TRAAK. Despite 78% of homology between TREK-1 and TREK-2, these two lipid- and mechano-activated K + channels are differently regulated. Recent studies reported that functional TREK-1 heterodimers are formed with TREK-2 and TRAAK channels ( Blin et al., 2016 ; Levitz et al., 2016 ). The heterodimer TREK-1-TREK-2 had minimal activity at physiological pH. However, TREK-1-TREK-2 complex is activated in both alkaline and acidic environments. TREK-1-TRAAK heterodimer shows activation by both intracellular acidification and alkalinization ( Levitz et al., 2016 ). Functional TREK-1-TRAAK heterodimers are formed and present unique biophysical properties and different type of regulations ( Blin et al., 2016 ). Heteromerization of TREK subfamily leads to diversification of K 2P channels and provides different kinds of regulations. Recently, it has been shown that a truncated form (TRESK-MT) of TRESK channel, a K 2P frequently associated with migraine, heteromerizes with and inhibits TREK-1 and TREK-2 ( Royal et al., 2019 ). The heterodimer TRESK-TREK acts as a dominant negative increasing trigeminal sensory neuron excitability and migraine-like phenotype in mice ( Royal et al., 2019 ).
Pharmacology of TREK-1 Channels
Trek-1 blockers.
Since the discovery of the involvement of TREK-1 in several CNS pathologies, very high interest has been devoted to find molecules modulators of its activity. As depicted in Table 1 , search for blockers of TREK-1 activity started with the discovery of the role of TREK-1 in depression processes. Mice model of depression lacking kcnk2 (the gene encoding for TREK-1) displays a phenotype resistant to the development of depression ( Heurteaux et al., 2006 ). Spadin was the first molecule that was developed in the aim to block TREK-1 channel and mimic the antidepressant-like phenotype of kcnk2 -/- mice ( Mazella et al., 2010 ; Djillani et al., 2018 ). Spadin is a peptide synthetized from the endogenous PE released after the cleavage of prosortilin by furin. Several analogs of spadin were developed afterward to improve spadin in vivo stability and antidepressant activity ( Veyssiere et al., 2015 ; Djillani et al., 2017 ). From a library of 22 peptides, the shorter peptide sequence PE 22–28 exhibited higher inhibition potency for TREK-1 associated with an improved stability in vivo and antidepressant properties ( Djillani et al., 2017 ). Similar properties were found in its biotinylated product or analog G/A-PE 22–28 (where Gly is replaced by an Ala). These spadin analogs show an extremely high affinity for TREK-1 as inhibition occurs at nanomolar concentrations. More interesting, spadin and analogs are specific blockers for TREK-1 as no effect was observed on both TREK-2 and TRAAK, the two closest K 2P members to TREK-1 which belong to the same subfamily and share almost 80% of homology ( Moha Ou Maati et al., 2012 ; Djillani et al., 2017 ).
TREK-1 blockers.
Selective Serotonin Reuptake Inhibitors (SSRI) is a family of small molecules with a high affinity for the serotonin transporter, SERT ( Hirano et al., 2005 ). SERT blockade generates an accumulation of serotonin in the synaptic clefts and displays antidepressant effects in clinic. In addition, at clinical concentrations, SSRI and fluoxetine antagonize TREK-1 channel (IC 50 = 19 μM for fluoxetine and IC 50 = 9 μM for Norfluoxetine, its active metabolite) ( Kennard et al., 2005 ). Other SSRIs such as paroxetine, citalopram or escitalopram also block TREK-1 with different potencies. However, SSRIs are not specific for TREK-1 since they target TREK-2, Nav1.5, and L-type Ca 2+ channels ( Wong et al., 2005 ; Poulin et al., 2014 ; Mcclenaghan et al., 2016 ).
Antipsychotic drugs used to treat psychosis, like schizophrenia and bipolar disorder, were shown to be potent blockers of TREK-1 channels. They antagonize TREK-1 and TREK-2 but do not affect TRAAK channels ( Table 1 ; Thummler et al., 2007 ). TREK-1 blockade was observed with both typical and atypical antipsychotic. For example, chlorpromazine and Loxapine block dose-dependently TREK-1 channels with IC 50 of 2.7 and 19.7 μM ( Thummler et al., 2007 ). These data demonstrate a potential link between TREK-1, schizophrenia and bipolar disorder.
Dihydropyridine analogs such as amlodipine or niguldipine are L-type Ca 2+ channel blockers. It has been shown that they also block TREK-1 with high affinity (IC 50 = 0.43 and 0.75 μM for amlodipine and niguldipine, respectively). In addition, mice lacking the L-type channel Cav1.3 display an antidepressant-like phenotype ( Busquet et al., 2010 ). The compound SID1900 blocks TREK-1 with an IC 50 ∼30 μM and was shown to produce antidepressant-like properties in a rat model of chronic unpredictable mild stress (CUMS) ( Ye et al., 2015 ). SID1900 effect was comparable to spadin. Still there is no evidence about other possible targets of SID1900 and more data are needed to conclude about its specificity. Finally, other molecules were reported to block TREK-1 channels and provide neuroprotection against ischemia and stroke such as 3-n-butylphtalide (NBP) and its analog lig4-4 ( Ji et al., 2011 ; Wang et al., 2018 ). However, if information is lacking about the specificity of NBP, lig4-4 was reported to affect hERG channel, voltage-gated K + channels (K v ), neuronal Na + and Ca 2+ channels, which could limit its development as potential neuroprotective molecule.
TREK-1 Activators
TREK-1 channel is activated by volatile general anesthetics such as chloroform, diethyl ether, halothane and isoflurane and it was shown that the C-terminal part is critical for its activation ( Table 2 ; Patel et al., 1999 ). If the chloroform seems to be selective for TREK-1 channels with an EC 50 (0.2–1.6 mM), halothane and isoflurane activate both TREK-1 and TASK channels. Furthermore, diethyl ether opens TREK-1 but decrease TASK channel activity ( Patel et al., 1999 ). In contrast to general anesthetics, local anesthetics like bupivacaine behave as inhibitors of TREK-1 ( Kindler et al., 1999 ). AA and other PUFAs open TREK-1 channels in dose-dependent manner ( Patel et al., 1998 ). Channel opening by AA, α-linolenic acid (ALA), and docosahexaenoic acid (DHA) is thought to contribute to neuroprotection ( Lauritzen et al., 2000 ). If SSRI antidepressants and antipsychotics drugs act as TREK-1 blockers, mood stabilizers such as lithium chloride and antiepileptics drugs like gabapentine, valproate and carbamazepine were reported to activate TREK-1 channel ( Kim et al., 2017 ). The tetrazole based compound BL-1249 showed an interesting TREK-1 activation with an EC 50 of around 1.5 μM in vitro in cultured human urinary bladder myocytes ( Tertyshnikova et al., 2005 ). In a pancreatic carcinoma cell line, BL-1249 activates dose-dependently TREK-1 with an IC 50 = 2 ± 2 μM ( Sauter et al., 2016 ). Recent studies provided more insights into the action mechanism of BL-1249. Indeed, it was shown that BL-1249 activates the selectivity filter C-type gate and activates selectively TREK-1 and TREK-2 channels 10-fold stronger than TRAAK channels ( Pope et al., 2018 ). In addition, BL-1249 action requires the C-terminal tail of the channel for the activation and the transmembrane domains M2 and M3 are critical for its selectivity ( Pope et al., 2018 ). Following the screening of more than 106,000 small molecules, an activator of TREK-1 named ML67 and its optimized analog ML67-33 were identified ( Bagriantsev et al., 2013 ). Then after, two molecules with higher potency were recently developed by the same laboratory, ML335 and ML402 which potentiate TREK-1 activity with EC 50 of 5.2 ± 0.8 μM and 5.9 ± 1.6 μM, respectively ( Table 2 ; Lolicato et al., 2017 ). ML335 and ML402 bind and activate a cryptic binding pocket within the C-type gate selectivity filter of TREK-1 channel ( Lolicato et al., 2017 ). Non-steroid anti-inflammatory drugs (NSAIDs) such as flufenamic acid, niflumic acid and mefenamic acid were reported to activate TREK-1 channels independently of cyclooxygenase COX inhibition ( Veale et al., 2014 ). Caffeic acid derivatives were reported to activates TREK-1 channel and provide potent analgesic effect in vivo ( Rodrigues et al., 2014 ; Vivier et al., 2017 ). Finally, small molecule GI-530159 was described as TREK-1 opener which reduces small DRG neurons excitability ( Loucif et al., 2018 ). However, GI-530159 is not selective for TREK-1 channels since it activates also TREK-2 with no significant effect on TRAAK channels ( Loucif et al., 2018 ).
TREK-1 activators.
TREK-1 Modulators
Riluzole ( Table 2 ) is a neuroprotective molecule marketed as an anticonvulsant drug. Its action mechanism involves the blockade of glutamate receptors. It is also prescribed to prolong the survival of patient suffering from amyotrophic lateral sclerosis. Besides, riluzole was shown to act as a transient TREK-1 activator within 30 s and strong inhibitor after 90 s ( Duprat et al., 2000 ). The dual activity on TREK-1 is thought to be mediated through cyclic Adenosine MonoPhosphate (cAMP) activation of PKA. On the other hand, riluzole produces a sustained activation of TRAAK without an inhibition.
TREK-1 and CNS Disorders
TREK-1 channels show a widespread distribution in rat and mouse brains ( Hervieu et al., 2001 ; Talley et al., 2001 ). It is rational to imagine multiple roles that TREK-1 can play in the CNS. In this part of the review, we will explain in depth why TREK-1 channel became a very promising target in numbers of pathologies that affect the CNS ( Figure 3 ).
TREK-1 in Depression
The role of TREK-1 in depression was demonstrated in mice invalidated for kcnk2 , the gene encoding for TREK-1 ( Heurteaux et al., 2006 ). TREK-1 knock-out mice displayed a phenotype resistant to depression in five different mouse models of depression: the Porsolt forced swim test (FST), the tail suspension test (TST), the conditioned suppression of motility test (CSMT), the learned helplessness test (LH), and the novelty-suppressed feeding test (NSF) ( Table 3 ; Heurteaux et al., 2006 ). The behavior of kcnk2 -/- mice was similar to those treated acutely or chronically with classical antidepressants like fluoxetine ( Heurteaux et al., 2006 ). Kcnk2 -deficient mice show an increase in serotonin (5-HT) neurotransmission in the DRN neurons. Deletion of TREK-1 increases neurogenesis induced with a chronic treatment with antidepressants ( Heurteaux et al., 2006 ). Blocking TREK-1 has become a novel strategy to design new generation of antidepressants. At the concentrations used clinically, SSRIs inhibits TREK-1 channels ( Kennard et al., 2005 ).
TREK-1 in CNS pathologies.
Spadin was previously described as a specific blocker of TREK-1 with a high affinity (IC 50 = 40–70 nM) ( Mazella et al., 2010 ; Djillani et al., 2017 , 2018 ). Spadin consists in 17-amino acid peptide synthesized from the propeptide (PE). PE is 44 amino acids generated from the maturation of sortilin by the cleavage of the prosortilin with furin ( Munck Petersen et al., 1999 ). Spadin binds with high affinities both TREK-1 and sortilin/neurotensin receptor 3 (NTSR3). Spadin by blocking TREK-1 generates mice with depression-resistance phenotype in several depression tests after only 4 days of treatment. Similarly to kcnk2 -/- mice, spadin enhances 5-HT neurotransmission in DRN serotonergic neurons ( Mazella et al., 2010 ). In in vitro experiments, spadin stimulates MAPK and PI3K pathways in time and concentration-dependent manner. Spadin at 100 nM increases the phosphorylation of ERK1/2 and Akt but does not affect the phosphorylation of mTOR, suggesting an original mechanism of action of spadin different from the other fast-acting antidepressant ketamine which was demonstrated to be mTOR-dependent ( Devader et al., 2015 ). Spadin has protective effects on neurons against staurosporine-induced Caspase-3 apoptosis through the specific activation of the PI3K pathway. Spadin increases transiently and after 8 h the mRNA expression of PSD-95 and synapsin, two markers of synaptogenesis in the brain. This increase is associated with a transient BDNF increase only after 5 h. Spadin increases also the proportion of mature spines in cortical neuronal culture. In vivo , daily spadin administration for 4 days increased mRNA expression of BDNF, PSD-95 and synapsin after only 7 days in the hippocampus. However, in the prefrontal cortex (PFC), only BDNF was enhanced after 3 weeks ( Devader et al., 2015 ).
The FST antidepressant activities of spadin disappear after 7 h after an acute treatment. To improve the bioavailability and the in vivo stability of spadin, retro-inverso analogs of spadin were designed and screened on the cell line stably expressing the human TREK-1 ( Veyssiere et al., 2015 ). Two analogs, 3 and 8, were identified. They prolong the antidepressant activity from 7 to 16 h in FST. However, due to some issues concerning in vitro toxicity of analog 3 and 8 at higher concentrations, other strategies were conducted in order to ameliorate the benefit/risk ratio. By shortening spadin sequence, a peptide PE 22-28 containing only 7 amino acids was identified as the shortest efficient molecule displaying an antidepressant activity ( Djillani et al., 2017 , 2018 ). The role of TREK-1 in mice was supported by other studies in human like STAR( ∗ )D which highlights a strong association between resistance to SSRIs and the existence of four single nucleotide polymorphisms (SNP) in kcnk2 gene ( Perlis et al., 2008 ). Another SNP, rs6686529 located in kcnk2 gene was found to be associated with major depression disorder and response to antidepressant treatment ( Liou et al., 2009 ). All these data highlight the key role of TREK-1 in depression and the need to design blockers of TREK-1 as promising original antidepressant ( Figure 3 ).
TREK-1 in Neuroprotection
Trek-1 in epileptogenesis.
TREK-1 is expressed in GABA-containing interneurons in the cortex and the hippocampus ( Hervieu et al., 2001 ). In vivo , It was reported that TREK-1 activators PUFA such as α-linolenic acid protect rats treated with the glutamate receptor agonist, kainic acid (KA) against seizures and hippocampal lesions ( Lauritzen et al., 2000 ). Moreover, PUFA neuroprotection was observed in another model of seizures using glutamatergic neurons. This effect was associated with the inhibition of the glutamatergic neurotransmission ( Lauritzen et al., 2000 ). TREK-1 deficient mice were shown to be more vulnerable to develop epileptic seizures triggered by KA and pentylenetetrazol (PTZ, GABA A receptor antagonist) ( Heurteaux et al., 2004 ). The expression of c-fos , a marker of neuronal excitability was increased in CA3 pyramidal neurons following treatment with KA ( Heurteaux et al., 2004 ). Linolenic acid and lysophosphatidylcholine (LPC) decrease KA-induced seizures in wild-type mice. However, no effect was observed in mice lacking TREK-1 channels ( Heurteaux et al., 2004 ). The protective effects of linolenic acid and LPC require the presence of TREK-1 channels. Status epilepticus (SE) is a persistent repeated seizure activity for more than 5 min. Development of TREK-1 mutant (TREK-M) produced a constitutively open TREK-1 channels that showed resistance to PKA and PKC downregulation. TREK-M was able to hyperpolarize the plasma membrane and decrease spontaneous firing of hippocampal neurons in culture ( Dey et al., 2014 ). In vivo administration of a recombinant adenoassociated virus (AAV)-mediated delivery of TREK-M in both entorhinal cortex and hippocampal CA3 regions, reduced by 50% the duration of SE in a mouse model of SE induced with lithium and pilocarpine. AAV-TREK-M prevented neuronal death in both entorhinal cortex and CA3 after injection ( Table 3 ; Dey et al., 2014 ). Since TREK-1 opening results in neuroprotection against epileptic episodes, blocking this AA-activated K 2P channel could in contrast be deleterious. Surprisingly, TREK-1 antagonist spadin does not enhance seizures induced by KA or PTZ when injected to mice. More interestingly, mice treated with spadin show more resistance to develop generalized convulsions and to induce death ( Moha Ou Maati et al., 2012 ; Djillani et al., 2018 ).
TREK-1 in ischemia
Using a model of global ischemia, results from transient bilateral occlusion of common carotid arteries in wild-type and knock-out TREK-1 mice showed that 74% of TREK -/- mice died 3 days after ischemia whereas only 34% died in wild-type group ( Heurteaux et al., 2004 ). Here again, linolenic acid or LPC pretreatment were unable to protect against ischemia in TREK-1 deficient mice in contrast to wild-type mice in which mice survival was significantly increased ( Heurteaux et al., 2004 ). Spinal cord ischemia is induced in mice by occluding both the aortic arch and left sub-clavian artery. It was reported that 75% of kcnk2 -/- mice died 3 h after 10 min ischemia compared to only 14% of wild-type mice 24 h after ischemia ( Heurteaux et al., 2004 ). Furthermore, surviving TREK-1 -/- mice developed severe hind limb paralysis. However, no neurological deficit was observed with wild-type mice ( Heurteaux et al., 2004 ). In addition to neurons, astrocytes were shown to play an important role in brain ischemia. TREK-1 contributes in maintaining the highly negative membrane potential in astrocytes which is crucial in controlling cell excitability ( Zhou et al., 2009 ). In cultured cortical astrocytes and in hippocampal slices, TREK-1 is widely expressed. Cortical and hippocampal TREK-1 channels were upregulated during astrogliosis following focal ischemia ( Wang et al., 2012 ). In hypoxic conditions, TREK-1 protein expression in astrocytes was also upregulated which increases glutamate clearance, suppressed the astrocytic marker S100β and block neuronal death ( Table 3 ; Wu et al., 2013 ). Glutamate is the main excitatory neurotransmitter in the brain. Astrocytes release glutamate following GPCR activation. DH Woo et al. demonstrate that TREK-1 channel is involved in the fast glutamate release which requires the interaction between the N-terminal domain of TREK-1 and the G βγ of the G αi PCR ( Woo et al., 2012 ).
TREK-1 in general anesthesia
In general anesthesia, at clinical doses used for isoflurane, diethyl ether, halothane and chloroform, TREK-1 channels are activated ( Patel et al., 1999 ). TREK-1 channel activation implies the C-terminal moiety and leads to cell hyperpolarization, decreased action potential firing and neuroprotection. The laughing gas nitrous oxide and xenon mostly exert their anesthetic effects via antagonizing NMDA receptors since they provide analgesia, euphoria and neuroprotection. They do not potentiate GABA A receptors ( Gruss et al., 2004 ). Nitrous oxide and xenon were shown to open TREK-1 channels with Glu306 as a critical amino acid for the anesthetic activation of TREK-1 ( Gruss et al., 2004 ). Chloral hydrate is used in pediatrics in certain forms of epilepsy such as progressive myoclonus and refractory childhood epilepsies. Upon administration, Chloral hydrate is rapidly metabolized into its active metabolite 2,2,2-trichlorethanol (TCE) which is thought to be responsible for the chloral hydrate activity ( Harinath and Sikdar, 2004 ). TCE induces depression of the CNS by potentiating GABA A receptors and inhibiting NMDA, AMPA, and Kainate receptors. It was reported that TCE also activates TREK-1 and TRAAK channels and contributes to the central anesthetic effect ( Harinath and Sikdar, 2004 ). In vivo , mice lacking TREK-1 channels displayed a lower sensitivity to the most potent TREK-1 activators, chloroform and halothane and also to sevoflurane, desflurane and isoflurane ( Heurteaux et al., 2004 ). The threshold to provoke anesthesia and the doses required for the anesthetic action were higher. Phenobarbital, a general anesthetic that does not modulate TREK-1 channels, fails to reproduce these effects in kcnk2 -/- mice. Thus, the observed effects were due specifically to TREK channel absence. Recently, in vitro and in vivo preconditioning with sevoflurane was shown to provide neuroprotection through activation of TREK-1 channels ( Tong et al., 2014 ). Rats treated with the anesthetic sevoflurane after MCAO display neuroprotection which seems to be mediated by TREK-1 channels ( Table 3 ; Pan et al., 2017 ).
Role of TREK-1 in Post-stroke Depression
Given the neuroprotective role of TREK-1 channels in stroke and its involvement in the depression process. It makes sense to address the role of this K 2P channel in the physiopathology of post-stroke depression (PSD). Recently, several laboratories have started to investigate the potential role of TREK-1 channels in this pathology. PSD is a high prevalence neuropsychiatric disorder that takes place after stroke onset. It has been shown that TREK-1 channel expression levels were elevated in the hippocampus and the PFC in rat model of PSD ( Table 3 ; Lin et al., 2015 ). Noteworthy, TREK-1 channel up-regulation was suppressed following chronic treatment with the TREK-1 blocker and the SSRI, escitalopram. In this PSD rat model, the main actors causing PSD in human patients were reproduced. For example, the middle cerebral artery occlusion (MCAO) surgery was set up to mimic stroke and chronic mild stress (CMS) and isolation rearing to mimic stress and psychosocial conditions, respectively ( Lin et al., 2015 ).
TREK-1 in Pain Perception
TREK-1 channels are known to be modulated by heat. Those K 2P channels are gradually and reversibly opened by temperature. Noteworthy, TREK-1 channel opening is reversed by cAMP and prostaglandin E2, two sensitizers of peripheral and central thermoreceptors. TREK-1 blockade is due to the phosphorylation of Ser333 on its C-terminal end. The thermosensitive TREK-1 channel is highly expressed and localized in small dorsal root ganglion (DRG) neurons and in central hypothalamic neurons, areas that are highly involved in pain ( Maingret et al., 2000 ). Sixty percent of sensory neurons express TREK-1 and most of them are associated with substance P. More than 40% of DRG neurons that express TREK-1 channels also express the thermal nociceptor TRPV1 channels. In vivo , compared to wild type mice, mice lacking kcnk2 gene are highly sensitive to low-threshold thermal pain and mechanical stimuli ( Alloui et al., 2006 ). TREK-1 deficient mice showed an exacerbated focal inflammatory response after spinal cord injury (SCI). Furthermore, TREK-1 deficiency enhanced astrogliosis, neuronal apoptosis, demyelination and retarded motor recovery ( Fang et al., 2017 ). Nevertheless, in other reported studies, in the DRG, the microRNA miR-183 expression was decreased and TREK-1 expression was increased in neuropathic pain induced by chronic constriction of sciatic nerve (CCI) ( Table 3 ; Han et al., 2016 ; Shi et al., 2018 ). Intrathecal injection of miR-183 alleviated pain in rat model of CCI and downregulated TREK-1 expression ( Shi et al., 2018 ). Recently, it was reported that TREK-1 modulator riluzole prevents both sensory and motor deficits in neuropathic pain induced by the chemotherapy drug oxaliplatin ( Poupon et al., 2018 ). Nevertheless, conclusions drawn by this paper are not totally convincing because authors only consider riluzole as an activator and do not take into account its inhibitory effects. Finally, TREK-2 and TRAAK channels, the other members of TREK subfamily are characterized in the small sized DRG neurons of rats ( Viatchenko-Karpinski et al., 2018 ).
Peripheral Roles of TREK-1 Channels
Trek-1 in the heart.
In the heart, the stretch-activated K + channels (SAK) repolarize cell membrane and counterbalance the activity of stretch-activated Cation channels (SAC) which in contrast increase cation influx and cell depolarization. TREK-1 channel presents a serious candidate to form SAK in heart with the large conductance Ca 2+ -activated K + channel (BK Ca ) and K ATP channels ( Decher et al., 2017a ). In rat heart, all K 2P channel genes were detected in at least one heart chamber with a prevalence of TWIK-2, TASK-1 and TREK-1 expression. TREK-1 channel was highly expressed in the right ventricle ( Liu and Saint, 2004 ). In rat neonatal atrial myocytes, using single channel inside-out patch, TREK-1-like current was activated by AA and internal acidosis ( Kim and Clapham, 1989 ). This TREK-1-like current was shown to be reversibly activated by volatile anesthetics such as chloroform, halothane and isoflurane. It was also downregulated by cAMP analogs and β-adrenergic receptor agonists ( Terrenoire et al., 2001 ). SAK current shared identical biophysical properties with the recombinant TREK-1 current such as the single channel conductance, no voltage-dependency and sensitivity to volatile anesthetics. In the ventricular cardiomyocytes, two variants of TREK-1 were found, large conductance and small conductance with (132 ± 5 pS) and (41 ± 5 pS), respectively, at positive potentials ( Xian Tao et al., 2006 ). The low-conductance TREK-1 variant was opened by mechanical stretch, internal acidification and AA. The biophysical properties of the two TREK-1 channels were similar to those displayed by the recombinant TREK-1 channels expressed in HEK293 cells. Using cardiac specific TREK-1 deficient mice, it was proposed that TREK-1 plays an essential role in sinoatrial node cell excitability ( Unudurthi et al., 2016 ). Recently, a heterozygous point mutation in the selectivity filter of TREK-1 channel was identified in patients diagnosed with right ventricular outflow tract (RVOT) tachycardia ( Decher et al., 2017b ). More interestingly, TREK-M showed a hypersensitivity to stretch and permeability to sodium ions ( Decher et al., 2017b ; Figure 3 ).
Role of TREK-1 in Other Tissues
In addition to the central roles of TREK-1 channels in the brain, it was thoroughly rational to think that modulating TREK-1 channels could play a peripheral role as this K 2P channel is expressed in number of tissues such as pancreas, prostate and smooth muscle cells ( Medhurst et al., 2001 ). The pancreas and particularly the insulin secreting β-cells constitute one of the main targets of the peripheral tissues where TREK-1 and other K 2P channels play an important role in glucose homeostasis ( Kang et al., 2004 ; Mazella et al., 2010 ; Dadi et al., 2014 ; Vierra et al., 2015 ). Given the importance of TREK-1 channel as a key modulator of plasma membrane potential in addition to its regulation by various physical and chemical stimuli, its role in the glucose homeostasis has been investigated using spadin ( Hivelin et al., 2016 ). Spadin inhibits TREK-1 channels in the pancreatic β-cell line β-TC3 ( Mazella et al., 2010 ). It is known that the regulation of insulin secretion by pancreatic β-cell is fine-tuned by a variety of hormones such as glucagon like-peptide-1 (GLP-1), leptin, estrogen, melatonin and growth hormone ( Fu et al., 2013 ). It is well established that ATP-sensitive potassium channels (K ATP ) play a critical role in maintaining glucose homeostasis ( Mctaggart et al., 2010 ). K ATP channels serve as a metabolic sensor for the pancreatic β-cell. When glucose levels increase, K ATP channels close, inducing a membrane depolarization resulting in the opening of Ca 2+ channels as well as the secretion of numerous hormones and amplification of the effects of glucose and other secretagogues such as GLP-1 ( Mctaggart et al., 2010 ). TREK-1 blockade by spadin induces insulin secretion only upon glucose stimulation. However, in contrast to incretin hormones, spadin induces Ca 2+ increase and insulin release through an original PKA-independent mechanism ( Hivelin et al., 2016 ). TREK-1 channels are inhibited as well by the PKA phosphorylation of the Ser333 at the C-terminal moiety of the channel ( Murbartián et al., 2005 ), which is the consequence of the activation of different G-protein-coupled receptors at the plasma membrane. By binding to their GLP-1 receptors, GLP-1 and its analogs such as exendin-4 are able to trigger cAMP synthesis and thus induce PKA activation leading to insulin secretion ( Göke et al., 1993 ). Then, insulin release by β-cells is potentiated by TREK-1 closing following PKA phosphorylation. Spadin or its analogs could substitute to incretin hormone actions and could propose a therapeutic alternative in case of inefficacy of antidiabetic treatment using GLP-1 mimetics. Among the other K 2P channels, the mechano-sensitive K 2P channel TREK-1 is predominately expressed in the human detrusor channel ( Andersson and Arner, 2004 ). Recently, a study assessing the relationship between six SNP and the occurrence of overactive lower urinary tract symptoms (LUTS), revealed a strong association between TREK-1 polymorphisms (rs758937019-CT genotype and rs758937019-T allele) and overactive LUTS in human ( Nedumaran et al., 2019 ).
Conclusion and Perspectives
Since their cloning 20 years ago, the physiological importance of TREK-1 channels has continued to grow ( Figure 3 ). Today, TREK-1 channels have been shown to be important and their presence is essential in a number of physiopathological processes. Their involvement in these different processes demonstrate the necessity to design pharmacological modulators, activators or inhibitors, of these channels to correct any TREK-1-related dysfunctions.
Despites a number of studies and many molecule screenings, only few putative new drugs were identified. The activators belonging to the ML and BL series show interesting results. However, they display lower affinities (in the micromolar range) and they are not specific for TREK-1 channels as they open other K 2P channels. TREK-1 openers are needed in pathologies such as ischemia, epilepsy and pain. The challenge is to improve their affinity, specificity and ability to cross the blood-brain barrier. The new ML and BL series of TREK- activators have not yet been tested in pathologies such epilepsy and ischemia. It would be interesting to investigate any neuroprotection effects of these molecules in rodent models of epilepsy or ischemia. In the same time, their specificity should be improved to avoid any off-target effects resulting from modulating other K 2P channels.
As inhibitors, the most promising molecules are spadin and its analogs. These peptides are very specific in the K 2P family and display very high affinities, in the picomolar range for the shorter analogs ( Djillani et al., 2017 ). Depression is a complex mood disorder where different actors are involved. TREK-1 is definitely one of the most promising ion channel that plays a key role in the neurobiology of depression. TREK-1 blockers such as spadin and short analogs display a huge therapeutic value over the classical antidepressants prescribed nowadays. They are fast-acting with highly affinity and specificity for TREK-1. More interestingly, they cross the blood-brain barrier which facilitates their further optimization and development as antidepressant drugs useful in clinic.
In the future, in addition to looking for new TREK-1 modulators to treat neuropsychiatric disorders, it is relevant to study the role of TREK-1 channels in peripheral tissues and understand their involvement in physiopathology. An emerging area where TREK-1 is displaying a growing interest is the heart. TREK-1 expression has been shown to change in cardiac hypertrophy ( Wang et al., 2013 ). Furthermore, a TREK-M was found to change channel permeability to sodium and cause ventricular tachycardia ( Decher et al., 2017b ).
To conclude, the wide distribution of TREK-1 in human body, the diverse stimuli and regulations that it could respond to, and the pleiotropic roles it could play in physiology and physiopathology, make TREK-1 a promising target to explore in depth but at the same time very challenging to design modulators that are tissue-specific.
Author Contributions
AD wrote the manuscript. MB, CH, and JM corrected it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Alloui A., Zimmermann K., Mamet J., Duprat F., Noel J., Chemin J., et al. (2006). TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25 2368–2376. 10.1038/sj.emboj.7601116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andersson K. E., Arner A. (2004). Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84 935–986. 10.1152/physrev.00038.2003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bagriantsev S. N., Ang K. H., Gallardo-Godoy A., Clark K. A., Arkin M. R., Renslo A. R., et al. (2013). A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS Chem. Biol. 8 1841–1851. 10.1021/cb400289x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barcia G., Fleming M. R., Deligniere A., Gazula V. R., Brown M. R., Langouet M., et al. (2012). De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 44 1255–1259. 10.1038/ng.2441 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barel O., Shalev S. A., Ofir R., Cohen A., Zlotogora J., Shorer Z., et al. (2008). Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am. J. Hum. Genet. 83 193–199. 10.1016/j.ajhg.2008.07.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bauer C. K., Calligari P., Radio F. C., Caputo V., Dentici M. L., Falah N., et al. (2018). Mutations in KCNK4 that affect gating cause a recognizable neurodevelopmental syndrome. Am. J. Hum. Genet. 103 621–630. 10.1016/j.ajhg.2018.09.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benatar M. (2000). Neurological potassium channelopathies. QJM 93 787–797. 10.1093/qjmed/93.12.787 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blin S., Ben Soussia I., Kim E. J., Brau F., Kang D., Lesage F., et al. (2016). Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc. Natl. Acad. Sci. U.S.A. 113 4200–4205. 10.1073/pnas.1522748113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brohawn S. G. (2015). How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 1352 20–32. 10.1111/nyas.12874 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brohawn S. G., Su Z., Mackinnon R. (2014). Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. U.S.A. 111 3614–3619. 10.1073/pnas.1320768111 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown D. A., Passmore G. M. (2009). Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156 1185–1195. 10.1111/j.1476-5381.2009.00111.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Busquet P., Nguyen N. K., Schmid E., Tanimoto N., Seeliger M. W., Ben-Yosef T., et al. (2010). CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 13 499–513. 10.1017/S1461145709990368 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chemin J., Patel A. J., Duprat F., Lauritzen I., Lazdunski M., Honore E. (2005). A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 24 44–53. 10.1038/sj.emboj.7600494 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen C., Wang L., Rong X., Wang W., Wang X. (2015). Effects of fluoxetine on protein expression of potassium ion channels in the brain of chronic mild stress rats. Acta Pharm. Sin. B 5 55–61. 10.1016/j.apsb.2014.12.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheramy A., Barbeito L., Godeheu G., Glowinski J. (1992). Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neurosci. Lett. 147 209–212. 10.1016/0304-3940(92)90597-Z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Comoglio Y., Levitz J., Kienzler M. A., Lesage F., Isacoff E. Y., Sandoz G. (2014). Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid. Proc. Natl. Acad. Sci. U.S.A. 111 13547–13552. 10.1073/pnas.1407160111 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dadi P. K., Vierra N. C., Jacobson D. A. (2014). Pancreatic β-cell-specific ablation of TASK-1 channels augments glucose-stimulated calcium entry and insulin secretion, improving glucose tolerance. Endocrinology 155 3757–3768. 10.1210/en.2013-2051 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Decher N., Kiper A. K., Rinne S. (2017a). Stretch-activated potassium currents in the heart: focus on TREK-1 and arrhythmias. Prog. Biophys. Mol. Biol. 130 223–232. 10.1016/j.pbiomolbio.2017.05.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Decher N., Ortiz-Bonnin B., Friedrich C., Schewe M., Kiper A. K., Rinne S., et al. (2017b). Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 9 403–414. 10.15252/emmm.201606690 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Devader C., Khayachi A., Veyssiere J., Moha Ou Maati H., Roulot M., Moreno S., et al. (2015). In vitro and in vivo regulation of synaptogenesis by the novel antidepressant spadin. Br. J. Pharmacol. 172 2604–2617. 10.1111/bph.13083 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dey D., Eckle V. S., Vitko I., Sullivan K. A., Lasiecka Z. M., Winckler B., et al. (2014). A potassium leak channel silences hyperactive neurons and ameliorates status epilepticus. Epilepsia 55 203–213. 10.1111/epi.12472 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Djillani A., Pietri M., Mazella J., Heurteaux C., Borsotto M. (2018). Fighting against depression with TREK-1 blockers: past and future. A focus on spadin. Pharmacol. Ther. 194 185–198. 10.1016/j.pharmthera.2018.10.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Djillani A., Pietri M., Moreno S., Heurteaux C., Mazella J., Borsotto M. (2017). Shortened spadin analogs display better TREK-1 inhibition, in vivo stability and antidepressant activity. Front. Pharmacol. 8 : 643 . 10.3389/fphar.2017.00643 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Du W., Bautista J. F., Yang H., Diez-Sampedro A., You S. A., Wang L., et al. (2005). Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37 733–738. 10.1038/ng1585 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duprat F., Lesage F., Patel A. J., Fink M., Romey G., Lazdunski M. (2000). The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 57 906–912. [ PubMed ] [ Google Scholar ]
- Esseltine J. L., Scott J. D. (2013). AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol. Sci. 34 648–655. 10.1016/j.tips.2013.10.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fang Y., Huang X., Wan Y., Tian H., Tian Y., Wang W., et al. (2017). Deficiency of TREK-1 potassium channel exacerbates secondary injury following spinal cord injury in mice. J. Neurochem. 141 236–246. 10.1111/jnc.13980 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fink M., Duprat F., Lesage F., Reyes R., Romey G., Heurteaux C., et al. (1996). Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 15 6854–6862. 10.1002/j.1460-2075.1996.tb01077.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fu Z., Gilbert E. R., Liu D. (2013). Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 9 25–53. 10.2174/1573399811309010025 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gloyn A. L., Pearson E. R., Antcliff J. F., Proks P., Bruining G. J., Slingerland A. S., et al. (2004). Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350 1838–1849. 10.1056/NEJMoa032922 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Göke R., Fehmann H. C., Linn T., Schmidt H., Krause M., Eng J., et al. (1993). Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting β-cells. J. Biol. Chem. 268 19650–19655. [ PubMed ] [ Google Scholar ]
- Gomez-Navarro N., Miller E. A. (2016). COP-coated vesicles. Curr. Biol. 26 R54–R57. 10.1016/j.cub.2015.12.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gruss M., Bushell T. J., Bright D. P., Lieb W. R., Mathie A., Franks N. P. (2004). Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharmacol. 65 443–452. 10.1124/mol.65.2.443 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Han H. J., Lee S. W., Kim G. T., Kim E. J., Kwon B., Kang D., et al. (2016). Enhanced Expression of TREK-1 Is related with chronic constriction injury of neuropathic pain mouse model in dorsal root ganglion. Biomol. Ther. 24 252–259. 10.4062/biomolther.2016.038 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harinath S., Sikdar S. K. (2004). Trichloroethanol enhances the activity of recombinant human TREK-1 and TRAAK channels. Neuropharmacology 46 750–760. 10.1016/j.neuropharm.2003.11.023 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hervieu G. J., Cluderay J. E., Gray C. W., Green P. J., Ranson J. L., Randall A. D., et al. (2001). Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 103 899–919. 10.1016/S0306-4522(01)00030-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heurteaux C., Guy N., Laigle C., Blondeau N., Duprat F., Mazzuca M., et al. (2004). TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 23 2684–2695. 10.1038/sj.emboj.7600234 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heurteaux C., Lucas G., Guy N., El Yacoubi M., Thummler S., Peng X. D., et al. (2006). Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 9 1134–1141. 10.1038/nn1749 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hirano K., Kimura R., Sugimoto Y., Yamada J., Uchida S., Kato Y., et al. (2005). Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br. J. Pharmacol. 144 695–702. 10.1038/sj.bjp.0706108 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hivelin C., Béraud-Dufour S., Devader C., Abderrahmani A., Moreno S., Moha Ou Maati H., et al. (2016). Potentiation of calcium influx and insulin secretion in pancreatic beta cell by the specific TREK-1 blocker spadin. J. Diabetes Res. 2016 : 3142175 . 10.1155/2016/3142175 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Honore E. (2007). The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8 251–261. 10.1038/nrn2117 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hubert J. P., Delumeau J. C., Glowinski J., Premont J., Doble A. (1994). Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br. J. Pharmacol. 113 261–267. 10.1111/j.1476-5381.1994.tb16203.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hwang E. M., Kim E., Yarishkin O., Woo D. H., Han K. S., Park N., et al. (2014). A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat. Commun. 5 : 3227 . 10.1038/ncomms4227 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ji X. C., Zhao W. H., Cao D. X., Shi Q. Q., Wang X. L. (2011). Novel neuroprotectant chiral 3-n-butylphthalide inhibits tandem-pore-domain potassium channel TREK-1. Acta Pharmacol. Sin. 32 182–187. 10.1038/aps.2010.210 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang D., Choe C., Kim D. (2004). Functional expression of TREK-2 in insulin-secreting MIN6 cells. Biochem. Biophys. Res. Commun. 323 323–331. 10.1016/j.bbrc.2004.08.089 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kennard L. E., Chumbley J. R., Ranatunga K. M., Armstrong S. J., Veale E. L., Mathie A. (2005). Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 144 821–829. 10.1038/sj.bjp.0706068 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim D., Clapham D. E. (1989). Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science 244 1174–1176. 10.1126/science.2727703 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E., Hwang E. M., Yarishkin O., Yoo J. C., Kim D., Park N., et al. (2010). Enhancement of TREK1 channel surface expression by protein-protein interaction with beta-COP. Biochem. Biophys. Res. Commun. 395 244–250. 10.1016/j.bbrc.2010.03.171 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E. J., Lee D. K., Hong S. G., Han J., Kang D. (2017). Activation of TREK-1, but Not TREK-2, channel by mood stabilizers. Int. J. Mol. Sci. 18 : E2460 . 10.3390/ijms18112460 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kindler C. H., Yost C. S., Gray A. T. (1999). Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology 90 1092–1102. 10.1097/00000542-199904000-00024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lafreniere R. G., Cader M. Z., Poulin J. F., Andres-Enguix I., Simoneau M., Gupta N., et al. (2010). A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 16 1157–1160. 10.1038/nm.2216 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauritzen I., Blondeau N., Heurteaux C., Widmann C., Romey G., Lazdunski M. (2000). Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 19 1784–1793. 10.1093/emboj/19.8.1784 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lei Q., Pan X. Q., Chang S., Malkowicz S. B., Guzzo T. J., Malykhina A. P. (2014). Response of the human detrusor to stretch is regulated by TREK-1, a two-pore-domain (K2P) mechano-gated potassium channel. J. Physiol. 592 3013–3030. 10.1113/jphysiol.2014.271718 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., et al. (1996). TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 15 1004–1011. 10.1002/j.1460-2075.1996.tb00437.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lesage F., Lazdunski M. (2000). Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Renal Physiol. 279 F793–F801. 10.1152/ajprenal.2000.279.5.F793 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levitz J., Royal P., Comoglio Y., Wdziekonski B., Schaub S., Clemens D. M., et al. (2016). Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl. Acad. Sci. U.S.A. 113 4194–4199. 10.1073/pnas.1522459113 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lin D. H., Zhang X. R., Ye D. Q., Xi G. J., Hui J. J., Liu S. S., et al. (2015). The role of the two-pore domain potassium channel TREK-1 in the therapeutic effects of escitalopram in a rat model of poststroke depression. CNS Neurosci. Ther. 21 504–512. 10.1111/cns.12384 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liou Y. J., Chen T. J., Tsai S. J., Yu Y. W., Cheng C. Y., Hong C. J. (2009). Support for the involvement of the KCNK2 gene in major depressive disorder and response to antidepressant treatment. Pharmacogenet. Genomics 19 735–741. 10.1097/FPC.0b013e32832cbe61 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu H., Enyeart J. A., Enyeart J. J. (2007). Potent inhibition of native TREK-1 K+ channels by selected dihydropyridine Ca2+ channel antagonists. J. Pharmacol. Exp. Ther. 323 39–48. 10.1124/jpet.107.125245 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu W., Saint D. A. (2004). Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin. Exp. Pharmacol. Physiol. 31 174–178. 10.1111/j.1440-1681.2004.03964.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lolicato M., Arrigoni C., Mori T., Sekioka Y., Bryant C., Clark K. A., et al. (2017). K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature 547 364–368. 10.1038/nature22988 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopes C. M., Rohacs T., Czirjak G., Balla T., Enyedi P., Logothetis D. E. (2005). PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J. Physiol. 564 117–129. 10.1113/jphysiol.2004.081935 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Loucif A. J. C., Saintot P. P., Liu J., Antonio B. M., Zellmer S. G., Yoger K., et al. (2018). GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K2P ) channel opener, reduces rat dorsal root ganglion neuron excitability. Br. J. Pharmacol. 175 2272–2283. 10.1111/bph.14098 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maingret F., Honore E., Lazdunski M., Patel A. J. (2002). Molecular basis of the voltage-dependent gating of TREK-1, a mechano-sensitive K(+) channel. Biochem. Biophys. Res. Commun. 292 339–346. 10.1006/bbrc.2002.6674 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maingret F., Lauritzen I., Patel A. J., Heurteaux C., Reyes R., Lesage F., et al. (2000). TREK-1 is a heat-activated background K(+) channel. EMBO J. 19 2483–2491. 10.1093/emboj/19.11.2483 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maingret F., Patel A. J., Lesage F., Lazdunski M., Honore E. (1999). Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 274 26691–26696. 10.1074/jbc.274.38.26691 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Martin D., Thompson M. A., Nadler J. V. (1993). The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur. J. Pharmacol. 250 473–476. 10.1016/0014-2999(93)90037-I [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazella J., Petrault O., Lucas G., Deval E., Beraud-Dufour S., Gandin C., et al. (2010). Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol. 8 : e1000355 . 10.1371/journal.pbio.1000355 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazella J., Zsürger N., Navarro V., Chabry J., Kaghad M., Caput D., et al. (1998). The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem. 273 26273–26276. 10.1074/jbc.273.41.26273 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mcclenaghan C., Schewe M., Aryal P., Carpenter E. P., Baukrowitz T., Tucker S. J. (2016). Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J. Gen. Physiol. 147 497–505. 10.1085/jgp.201611601 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McTaggart J. S., Clark R. H., Ashcroft F. M. (2010). The role of the K ATP channel in glucose homeostasis in health and disease: more than meets the islet. J. Physiol. 588 3201–3209. 10.1113/jphysiol.2010.191767 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Medhurst A. D., Rennie G., Chapman C. G., Meadows H., Duckworth M. D., Kelsell R. E., et al. (2001). Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res. Mol. Brain Res. 86 101–114. 10.1016/S0169-328X(00)00263-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moha Ou Maati H., Veyssiere J., Labbal F., Coppola T., Gandin C., Widmann C., et al. (2012). Spadin as a new antidepressant: absence of TREK-1-related side effects. Neuropharmacology 62 278–288. 10.1016/j.neuropharm.2011.07.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Munck Petersen C., Nielsen M. S., Jacobsen C., Tauris J., Jacobsen L., Gliemann J., et al. (1999). Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 18 595–604. 10.1093/emboj/18.3.595 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murbartián J., Lei Q., Sando J. J., Bayliss D. A. (2005). Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J. Biol. Chem. 280 30175–30184. 10.1074/jbc.M503862200 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nedumaran B., Pineda R. H., Rudra P., Lee S., Malykhina A. P. (2019). Association of genetic polymorphisms in the pore domains of mechano-gated TREK-1 channel with overactive lower urinary tract symptoms in humans. Neurourol. Urodyn. 38 144–150. 10.1002/nau.23862 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pan L., Yang F., Lu C., Jia C., Wang Q., Zeng K. (2017). Effects of sevoflurane on rats with ischemic brain injury and the role of the TREK-1 channel. Exp. Ther. Med. 14 2937–2942. 10.3892/etm.2017.4906 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel A. J., Honore E., Lesage F., Fink M., Romey G., Lazdunski M. (1999). Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 2 422–426. 10.1038/8084 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel A. J., Honore E., Maingret F., Lesage F., Fink M., Duprat F., et al. (1998). A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17 4283–4290. 10.1093/emboj/17.15.4283 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perlis R. H., Moorjani P., Fagerness J., Purcell S., Trivedi M. H., Fava M., et al. (2008). Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR( ∗ )D study. Neuropsychopharmacology 33 2810–2819. 10.1038/npp.2008.6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Petersen C. M., Nielsen M. S., Nykjær A., Jacobsen L., Tommerup N., Rasmussen H. H., et al. (1997). Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem. 272 3599–3605. 10.1074/jbc.272.6.3599 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pietrobon D. (2002). Calcium channels and channelopathies of the central nervous system. Mol. Neurobiol. 25 31–50. 10.1385/MN:25:1:031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pope L., Arrigoni C., Lou H., Bryant C., Gallardo-Godoy A., Renslo A. R., et al. (2018). Protein and Chemical Determinants of BL-1249 Action and Selectivity for K2P Channels. ACS Chem. Neurosci. 10.1021/acschemneuro.8b00337 [Epub ahead of print]. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poulin H., Bruhova I., Timour Q., Theriault O., Beaulieu J. M., Frassati D., et al. (2014). Fluoxetine blocks Nav1.5 channels via a mechanism similar to that of class 1 antiarrhythmics. Mol. Pharmacol. 86 378–389. 10.1124/mol.114.093104 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poupon L., Lamoine S., Pereira V., Barriere D. A., Lolignier S., Giraudet F., et al. (2018). Targeting the TREK-1 potassium channel via riluzole to eliminate the neuropathic and depressive-like effects of oxaliplatin. Neuropharmacology 140 43–61. 10.1016/j.neuropharm.2018.07.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rennolds J., Tower C., Musgrove L., Fan L., Maloney K., Clancy J. P., et al. (2008). Cystic fibrosis transmembrane conductance regulator trafficking is mediated by the COPI coat in epithelial cells. J. Biol. Chem. 283 833–839. 10.1074/jbc.M706504200 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rodrigues N., Bennis K., Vivier D., Pereira V., Chatelain C. F., Chapuy E., et al. (2014). Synthesis and structure-activity relationship study of substituted caffeate esters as antinociceptive agents modulating the TREK-1 channel. Eur. J. Med. Chem. 75 391–402. 10.1016/j.ejmech.2014.01.049 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Royal P., Andres-Bilbe A., Avalos Prado P., Verkest C., Wdziekonski B., Schaub S., et al. (2019). Migraine-associated TRESK mutations increase neuronal excitability through alternative translation initiation and inhibition of TREK. Neuron 101 : e236 . 10.1016/j.neuron.2018.11.039 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sandoz G., Tardy M. P., Thummler S., Feliciangeli S., Lazdunski M., Lesage F. (2008). Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J. Neurosci. 28 8545–8552. 10.1523/JNEUROSCI.1962-08.2008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sandoz G., Thummler S., Duprat F., Feliciangeli S., Vinh J., Escoubas P., et al. (2006). AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J. 25 5864–5872. 10.1038/sj.emboj.7601437 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Santos R., Ursu O., Gaulton A., Bento A. P., Donadi R. S., Bologa C. G., et al. (2017). A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16 19–34. 10.1038/nrd.2016.230 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sauter D. R., Sorensen C. E., Rapedius M., Bruggemann A., Novak I. (2016). pH-sensitive K(+) channel TREK-1 is a novel target in pancreatic cancer. Biochim. Biophys. Acta 1862 1994–2003. 10.1016/j.bbadis.2016.07.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schewe M., Nematian-Ardestani E., Sun H., Musinszki M., Cordeiro S., Bucci G., et al. (2016). A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K(+) Channels. Cell 164 937–949. 10.1016/j.cell.2016.02.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shi D. N., Yuan Y. T., Ye D., Kang L. M., Wen J., Chen H. P. (2018). MiR-183-5p alleviates chronic constriction injury-induced neuropathic pain through inhibition of TREK-1. Neurochem. Res. 43 1143–1149. 10.1007/s11064-018-2529-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Song J. H., Huang C. S., Nagata K., Yeh J. Z., Narahashi T. (1997). Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 282 707–714. [ PubMed ] [ Google Scholar ]
- Spillane J., Kullmann D. M., Hanna M. G. (2016). Genetic neurological channelopathies: molecular genetics and clinical phenotypes. J. Neurol. Neurosurg. Psychiatry 87 37–48. 10.1136/jnnp-2015-311233 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takahira M., Sakurai M., Sakurada N., Sugiyama K. (2005). Fenamates and diltiazem modulate lipid-sensitive mechano-gated 2P domain K(+) channels. Pflugers Arch. 451 474–478. 10.1007/s00424-005-1492-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talley E. M., Solorzano G., Lei Q., Kim D., Bayliss D. A. (2001). Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 21 7491–7505. 10.1523/JNEUROSCI.21-19-07491.2001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Terrenoire C., Lauritzen I., Lesage F., Romey G., Lazdunski M. (2001). A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ. Res. 89 336–342. 10.1161/hh1601.094979 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tertyshnikova S., Knox R. J., Plym M. J., Thalody G., Griffin C., Neelands T., et al. (2005). BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: a putative potassium channel opener with bladder-relaxant properties. J. Pharmacol. Exp. Ther. 313 250–259. 10.1124/jpet.104.078592 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thummler S., Duprat F., Lazdunski M. (2007). Antipsychotics inhibit TREK but not TRAAK channels. Biochem. Biophys. Res. Commun. 354 284–289. 10.1016/j.bbrc.2006.12.199 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tong L., Cai M., Huang Y., Zhang H., Su B., Li Z., et al. (2014). Activation of K(2)P channel-TREK1 mediates the neuroprotection induced by sevoflurane preconditioning. Br. J. Anaesth. 113 157–167. 10.1093/bja/aet338 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Unudurthi S. D., Wu X., Qian L., Amari F., Onal B., Li N., et al. (2016). Two-Pore K+ Channel TREK-1 regulates sinoatrial node membrane excitability. J. Am. Heart Assoc. 5 : e002865 . 10.1161/JAHA.115.002865 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Veale E. L., Al-Moubarak E., Bajaria N., Omoto K., Cao L., Tucker S. J., et al. (2014). Influence of the N terminus on the biophysical properties and pharmacology of TREK1 potassium channels. Mol. Pharmacol. 85 671–681. 10.1124/mol.113.091199 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Veyssiere J., Moha Ou Maati H., Mazella J., Gaudriault G., Moreno S., Heurteaux C., et al. (2015). Retroinverso analogs of spadin display increased antidepressant effects. Psychopharmacology 232 561–574. 10.1007/s00213-014-3683-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Viatchenko-Karpinski V., Ling J., Gu J. G. (2018). Characterization of temperature-sensitive leak K(+) currents and expression of TRAAK, TREK-1, and TREK2 channels in dorsal root ganglion neurons of rats. Mol. Brain 11 : 40 . 10.1186/s13041-018-0384-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vierra N. C., Dadi P. K., Jeong I., Dickerson M., Powell D. R., Jacobson D. A. (2015). Type 2 diabetes–associated K + channel TALK-1 modulates β-cell electrical excitability, second-phase insulin secretion, and glucose homeostasis. Diabetes 64 3818–3828. 10.2337/db15-0280 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vivier D., Soussia I. B., Rodrigues N., Lolignier S., Devilliers M., Chatelain F. C., et al. (2017). Development of the first two-pore domain potassium channel TWIK-related K(+) channel 1-selective agonist possessing in vivo antinociceptive activity. J. Med. Chem. 60 1076–1088. 10.1021/acs.jmedchem.6b01285 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang M., Song J., Xiao W., Yang L., Yuan J., Wang W., et al. (2012). Changes in lipid-sensitive two-pore domain potassium channel TREK-1 expression and its involvement in astrogliosis following cerebral ischemia in rats. J. Mol. Neurosci. 46 384–392. 10.1007/s12031-011-9598-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang W., Liu D., Xiao Q., Cai J., Feng N., Xu S., et al. (2018). Lig4-4 selectively inhibits TREK-1 and plays potent neuroprotective roles in vitro and in rat MCAO model. Neurosci. Lett. 671 93–98. 10.1016/j.neulet.2018.02.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang W., Zhang M., Li P., Yuan H., Feng N., Peng Y., et al. (2013). An increased TREK-1-like potassium current in ventricular myocytes during rat cardiac hypertrophy. J. Cardiovasc. Pharmacol. 61 302–310. 10.1097/FJC.0b013e318280c5a9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weckhuysen S., Mandelstam S., Suls A., Audenaert D., Deconinck T., Claes L. R., et al. (2012). KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 71 15–25. 10.1002/ana.22644 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wong D. T., Perry K. W., Bymaster F. P. (2005). Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 4 764–774. 10.1038/nrd1821 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woo D. H., Han K. S., Shim J. W., Yoon B. E., Kim E., Bae J. Y., et al. (2012). TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151 25–40. 10.1016/j.cell.2012.09.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu X., Liu Y., Chen X., Sun Q., Tang R., Wang W., et al. (2013). Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. J. Mol. Neurosci. 49 499–506. 10.1007/s12031-012-9875-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xian Tao L., Dyachenko V., Zuzarte M., Putzke C., Preisig-Muller R., Isenberg G., et al. (2006). The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 69 86–97. 10.1016/j.cardiores.2005.08.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu X., Pan Y., Wang X. (2004). Alterations in the expression of lipid and mechano-gated two-pore domain potassium channel genes in rat brain following chronic cerebral ischemia. Brain Res. Mol. Brain Res. 120 205–209. 10.1016/j.molbrainres.2003.09.020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ye D., Li Y., Zhang X., Guo F., Geng L., Zhang Q., et al. (2015). TREK1 channel blockade induces an antidepressant-like response synergizing with 5-HT1A receptor signaling. Eur. Neuropsychopharmacol. 25 2426–2436. 10.1016/j.euroneuro.2015.09.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou M., Xu G., Xie M., Zhang X., Schools G. P., Ma L., et al. (2009). TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29 8551–8564. 10.1523/JNEUROSCI.5784-08.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
COMMENTS
KCNK2. Potassium channel subfamily K member 2, also known as TREK-1, is a protein that in humans is encoded by the KCNK2 gene. [5] [6] [7] This gene encodes K 2P 2.1, a lipid-gated ion channel belonging to the two-pore-domain background potassium channel protein family. This type of potassium channel is formed by two homodimers that create a ...
Star Trek Natures Hunger: "Scorned at the Captain's Table" — Season 7, Episode 1. Posted on December 31, 2015. Posted in Fan Film.
This UV light-induced thermal hypersensitivity was prevented by co-application of LAKI with ML67.33, a specific TREK channel agonist 23,24, supporting TREK channel involvement (P > 0.21) (Fig. 3g).
Mechano-regulation of TREK channels. MS ion channels can be activated by two different mechanisms. The mechanism called tethering needs several cytoskeletal proteins as scaffold proteins to activate the mechano-sensor, this is the case of TRP channels ().The other mechanism implies the activation of the channels by the tension in the bilayer itself, without the need for other cellular ...
Therefore, targeting TREK channel intrinsic activity to reduce TG neuron excitability should be considered as an alternative strategy to treat migraine. Limitations of the study. This study was carried out in rodent models exhibiting an allodynic phenotype which is commonly used as a readout for the migraine-like phenotype studies in rodents ...
In this situation both channel types, if coexpressed, would tend to drive the cold receptor far from the threshold and hence we would expect the receptor to be hyperpolarized and silent. When the temperature is reduced (from 30 to 10°C), TREK channel activity would fall to zero and the activity of cold sensitive TRPs would strongly increase.
TREK channel regulation by G-protein signaling and regulatory protein partners. The neurotransmitters released upon noxious stimuli act on many metabotropic receptors expressed in nociceptive sensory neurons. Once activated, these receptors activate G-protein signaling, leading to the activation of phospholipases and protein kinases which ...
Season 5 of "Star Trek: Discovery" brings back new and old faces along with recurring guest stars. Cast members include: Sonequa Martin-Green as Captain Michael Burnham. Doug Jones as Saru ...
Star Trek: Discovery is available to stream for free on TVNZ+. You'll need to create a free account to start streaming. In addition to new season 5 episodes, Seasons 1-4 are also streaming on the ...
Star Trek: Discovery occupies an interesting place in the celebrated franchise. It was the first Trek series of the streaming era, the first to debut behind a paywall, the first produced after J.J ...
Gene heterogeneity. TREK-1 (K 2p 2.1), 6 TREK-2 (K 2p 10.1), 17, 18 and TRAAK (TWIK Related Arachidonic acid Activated K + channel) (K 2p 4.1), 19 compose the TREK subfamily of K 2p channels. TREK-1 shares 63% identity and 78% homology with TREK-2. The identity falls to 45% and homology to 69% with TRAAK and to 50-55% homology with the other K 2P subunits. 17, 18 Recent findings have ...
PlutoTV has been streaming Star Trek: The Original Series, The Next Generation, Deep Space Nine, and Voyager on those two live Star Trek channels. This week, PlutoTV launched a third channel in ...
This idea is supported by our own data in alveolar epithelial cells (Fig. 5) showing that TREK-1 currents can readily be induced by our channel activators ML335 and BL1249 44,46,47, thus making ...
In this review, we will focus on describing the role of one of the most studied K 2P channel, TREK-1 channel in health and disease. In addition to discussing the recent pharmacological modulators of its activity. Trek-1 Channel. TREK-1, named KCNK2 or K 2P 2.1 belongs to a large family of K 2P channels containing 15 members grouped in six ...
The TREK-1 channel is a temperature-sensitive, osmosensitive and mechano-gated K+ channel with a regulation by Gs and Gq coupled receptors. This paper demonstrates that TREK-1 qualifies as one of the molecular sensors involved in pain perception. TREK-1 is highly expressed in small sensory neurons, is present in both peptidergic and ...
"Strange New Worlds" is the 12th "Star Trek" TV show since the original series debuted on NBC in 1966, introducing Gene Roddenberry's vision of a hopeful future for humanity.
The Star Trek channel features the original series and The Next Generation while the More Star Trek channel features Voyager and Deep Space Nine, though now it's likely to focus solely on Voyager ...
Twik-related K + channel 1 (TREK1), TREK2, and Twik-related arachidonic-acid stimulated K + channel (TRAAK) are two-pore-domain K + (K 2P) ion channels that belong to the TREK channel subfamily and assemble as dimers to produce an inhibitory, outwardly rectifying potassium current.They are not very active under basal conditions but can be dynamically stimulated by a wide range of stimuli ...
Star Trek: Discovery season 5 brought back the 32nd century Starfleet's tribute to Captain Jonathan Archer (Scott Bakula) from Star Trek: Enterprise.Star Trek: Discovery and Star Trek: Enterprise are TV series at opposite points bookending Star Trek's Prime Universe timeline.Enterprise is set in the 22nd century and charts the pioneering voyages of the NX-01, the first Starship Enterprise ...
From a functional point of view, TREK-1 plays a critical role in countering the depolarizing effect of mechano-activated cationic currents, contributing to stimulation-activated central (heart) feedback mechanics in the cardiovascular system ().TREK-1 channels also have a potential role in regulating the normal activity of sinoatrial node-hosted pacemakers by preventing the occurrence of ...
To understand the mechanisms of polymodal K2P channel gating and inhibition by drugs, we solved the crystal structure of human TREK-2 in two conformations at 3.4 and 3.9 Å resolution (figs. S1 and S2 and table S1).The truncated protein used for crystallization retains many functional properties exhibited by wild-type TREK-2, including activation by stretch and AA and inhibition by ...
The Elecard company took part in the large-scale project on content preparation and broadcasting of 12 channels in the Moscow underground trains.
TREK-1 is a two-pore-domain background potassium channel expressed throughout the central nervous system. It is opened by polyunsaturated fatty acids and lysophospholipids. It is inhibited by neurotransmitters that produce an increase in intracellular cAMP and by those that activate the Gq protein pathway.
Gene heterogeneity. TREK-1 (K 2p 2.1), Citation 6 TREK-2 (K 2p 10.1), Citation 17, Citation 18 and TRAAK (TWIK Related Arachidonic acid Activated K + channel) (K 2p 4.1), Citation 19 compose the TREK subfamily of K 2p channels. TREK-1 shares 63% identity and 78% homology with TREK-2. The identity falls to 45% and homology to 69% with TRAAK and to 50-55% homology with the other K 2P subunits.
The 9th radio centre of Moscow was a high power shortwave and medium wave broadcasting facility at Elektrostal near Moscow.Its broadcasting frequency was 873 kHz with a transmission power of up to 1200 kilowatts. It was also used as radio jammer of "unwanted" stations.
40 Facts About Elektrostal. Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to ...
TREK-1 is the most studied background K 2P channel. Its main role is to control cell excitability and maintain the membrane potential below the threshold of depolarization. TREK-1 is multi-regulated by a variety of physical and chemical stimuli which makes it a very promising and challenging target in the treatment of several pathologies.