- Douleur chronique : définition, origine et traitement
- Centre anti-douleur : définition et liste complète
Centre anti douleur Tours : Chru Bretonneau - Tours pour la douleur chronique
Douleur chronique au centre anti douleur à Tours : Le centre CEDT CHRU Bretonneau à Tours, dirigé par le Dr Anne Philippe, prend en charge le traitement des douleurs chroniques. Informations sur le centre et son équipe médicale.

CHRU BRETONNEAU - TOURS : centre de traitement de la douleur chronique à Tours
A quoi sert le centre anti-douleur de tours.
Un centre de traitement de la douleur chronique aussi appelé Centre d'Evaluation et de Traitement de la Douleur ou CEDT (par exemple : CHRU BRETONNEAU - TOURS) est un organisme médical au sein duquel sont accueillies les patients qui souffrent de douleurs chroniques. Ces lieux, tels qu'à Tours , ont pour but de jauger votre douleur, de diagnostiquer d'éventuelles pathologies ou raisons physiques pouvant les causer, et de les traiter. Ces traitements comprennent souvent un traitement chimique, pouvant être accompagné de techniques pour apprendre à gérer sa douleur. Toutefois, le traitement de la douleur chronique en France reste contrainte par le nombre de docteurs et le temps pouvant être octroyé à chaque personne, ainsi que les types de thérapies mises en oeuvre. En outre, le temps d’attente pour être accueilli dans un CEDT dépasse la plus part du temps 6 mois.
CHRU BRETONNEAU - TOURS
Ce centre anti-douleur est localisé sur Tours. {{cedt-component}}
Origine de la douleur chronique
La douleur chronique est souvent définie comme perdurant depuis au minimum 3 mois. Celle-ci peut cibler chaque partie du corps humain. La douleur , lorsqu'elle est chronique, est souvent aggravée par divers symptômes tels que la fatigue, la dépression, les troubles du sommeil, ou alors l'anxiété. Les douleurs ,de surcroît si elles s'avèrent chroniques, peuvent pour certaines être générées par un évènement sous-jacent, comme une pathologie inflammatoire ou une anomalie structurelle, et il s'avère souhaitable de passer par les vérifications médicales obligatoires selon les instructions d'un praticien spécialisé dans ce domaine, entre autres lors d'une visite à un centre spécialisé dans les douleurs chroniques . Cependant, fréquemment chez les personnes souffrant de douleur chronique, aucune raison physique n’est décelée. Des études ces dernières années, ont effectivement démontré que l'étiologie de ces douleurs se trouve régulièrement dans notre cerveau. Quand nous souffrons d'une douleur, des voies neuronales se mettent en place au sein même de notre cerveau, tout cela pour rendre moins désagréable l'expérience suivante. Pour une majorité d' individus, lesdites voies restent inertes {tant} qu’aucune lésion ne se manifeste, donc les douleurs apparaissent de façon ordinaire dès lors qu'un tel évènement apparaît. A l'inverse, pour la plus grande partie des individus qui souffrent de douleurs, les chemins neuronaux s'avèrent être actifs alors qu'il n'y a aucune lésion. De fait, cette douleur a été mémorisée par notre cerveau, qui la manifeste de manière “erronée”. Ainsi, plus on souffre de douleurs chroniques, plus ces chemins neuronaux “se consolident”. Cette douleur chronique semble devenir ordinaire, comme la marche ou bien la bicyclette .
[ville] - Alternative au CEDT ?
Le suivi d'un individu qui souffre de douleurs chroniques doit continuellement être fondé sur la compréhension. Le suivi doit être conçu pour chaque individu et son parcours avec les douleurs chroniques . Ce sont ces bases fondamentales que je fais en sorte de mettre en oeuvre pour guider les personnes qui souffrent de douleurs chroniques. Dans cette optique, je mets en oeuvre les méthodes et thérapies m'ayant aidé à réduire mes douleurs chroniques. J'emploie par exemple une méthodologie ayant pour fonction de rendre inerte les chemins neuronaux de la douleur chronique lorsque cette réaction est “erronée”. Cette pratique, qui a particulièrement fait l’objet d’une revue scientifique publiée en 2021, est particulièrement efficace dans la prise en charge de la douleur. Je suis en outre hypnothérapeute afin de mener un travail sur le vécu de la personne. Par ailleurs, je mets en oeuvre diverses techniques afin de réaliser ce travail sur les émotions, telles que les études du docteur Sarno sur les émotions inconscientes, et leur lien avec les douleurs chroniques . Je propose des consultations notamment pour :
- la douleur cervicale
- la douleur de dos
- les maux de tête
- les fibromyalgies et leurs douleurs
- le mal de ventre
Pour les personnes sur Tours et ses alentours, je reçois dans mon cabinet situé à Joué-lès-Tours, mais propose aussi un suivi en visio ou à domicile.
<link rel="canonical" href="https://www.douleur-chronique.net/" />

La douleur chronique est une souffrance qui s'étend ou s'aggrave sur une durée longue. Elle est en général reconnue comme chronique si elle persiste au-delà de trois à six mois, bien que cela puisse varier en fonction des interprétations.
Le Centre de douleur chronique Chru Bretonneau - Tours est un établissement médical dédié à l'analyse, la détection et la gestion des douleurs persistantes, souvent plus de 3 à 6 mois, qui ne réagissent pas aux thérapies classiques.
Le Chru Bretonneau - Tours, un centre anti-douleur de référence, est situé à l'adresse suivante : 2 boulevard tonnelle, 37044 Tours.
Chru Bretonneau - Tours : solution conventionnelle contre la douleur chronique
Pour soigner une douleur chronique à Tours, n'hésitez pas à consulter au Chru Bretonneau - Tours, localisé au 2 boulevard tonnelle, 37044 Tours. Ce centre renommé propose une approche intégrale et des soins personnalisés pour les patients atteints de douleurs constantes.
Psychopraticien spécialisé : solution alternative au centre anti-douleur à Tours
Spécialiste douleur chronique, je suis psychopraticien et praticien en hypnose.
Je suis certifié en thérapie de retraitement de la douleur. J'accompagne les personnes souffrant de douleur :
- à Tours et ses environs en présentiel
- dans toute la France, y compris pour traiter la douleur chronique à Tours , en visio.
Mes services incluent notamment la prise en charge du mal de dos, des douleurs cervicales, des douleurs au ventre, des migraines, des symptômes de la fibromyalgie.

Vous souhaitez être accompagné dans votre gestion de la douleur chronique ?
Ensemble, nous travaillerons à :
- Comprendre les racines de votre douleur
- Identifier des techniques de gestion personnalisées
- Construire un chemin vers le soulagement de vos douleurs
Vous trouverez ci-après les autres centres traitant de la douleur chronique en Centre-Val de Loire.

Centre Hospitalier Dreux
Le CEDT du Centre Hospitalier Dreux, dirigé par le Dr François CARRE, propose des traitements pour les douleurs chroniques. Informations et prise en charge à Dreux.

Centre Hospitalier Chateauroux
Le traitement de la douleur chronique à Châteauroux est pris en charge par le CEDT du centre hospitalier. Le Dr Emmanuel CARREEL est le médecin référent du centre. Informations sur les traitements de la douleur chronique à Châteauroux.

Centre Hospitalier Chartres
Le CEDT du Centre Hospitalier de Chartres assure le traitement des douleurs chroniques à Coudray. Découvrez le Dr Mouldi HAMROUNI, médecin référent du centre.

Centre Hospitalier de Vendôme
Le CEDT du Centre Hospitalier de Vendôme, dirigé par le Dr B. LAFON, assure la prise en charge des douleurs chroniques à Vendôme. Informations sur les traitements et les soins disponibles.

Centre Hospitalier Blois
Le CEDT du Centre Hospitalier de Blois, sous la direction du Dr Benoit Lafon, propose des traitements pour la douleur chronique. Informations et prise en charge à Blois.

Chro - Nouvel Hôpital D’orléans
Le CEDT CHRO - Nouvel Hôpital d’Orléans est le centre de référence pour le traitement des douleurs chroniques à Orléans. Le Dr Isabelle ROUBY-LANDRIEUX est le médecin référent du centre. Obtenez des soins de qualité pour soulager vos douleurs chroniques.

Centre Hospitalier Bourges - Jacques Coeur
Le CEDT du Centre Hospitalier Jacques Coeur de Bourges assure le traitement de la douleur chronique. Dr Daniel Gerber est le médecin référent du centre.
Chru Bretonneau - Tours
Le centre CEDT CHRU Bretonneau à Tours, dirigé par le Dr Anne Philippe, prend en charge le traitement des douleurs chroniques. Informations sur le centre et son équipe médicale.

Centre Hospitalier Romorantin Lanthenay
Le CEDT du Centre Hospitalier ROMORANTIN LANTHENAY, dirigé par le Dr B. LAFON, assure le traitement des douleurs chroniques à Romorantin. Obtenez des informations sur les services proposés pour soulager la douleur chronique.
Conciergerie de CHRU Tours - Bretonneau

Comment ça marche ?
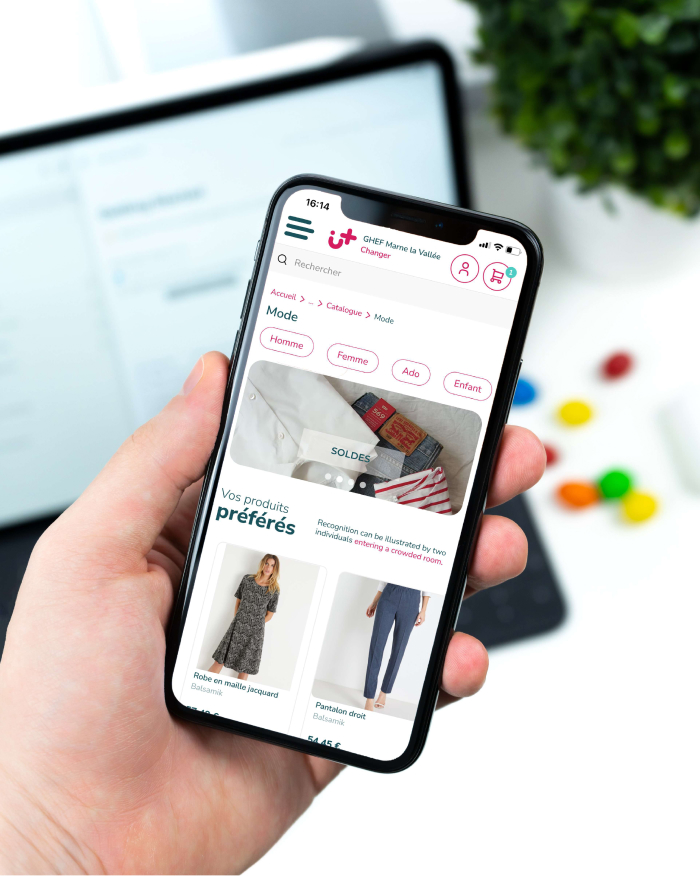
Découvrez les différents univers de produits grâce au menu en haut à gauche puis cliquez pour voir l’ensemble des produits et services proposés. Vous pouvez aussi découvrir les produits selon des spécialités : maternité, pédiatrie ou cancérologie.
2 Commandez
Choisissez les produits qui vous plaisent et ajoutez-les au panier : divertissement, vêtements, alimentaire, accessoires et même prestations bien-être ! Tout y est ! Une fois le panier finalisé, vous n’avez plus qu’à payer en ligne.

3 Récupérez
Soit vos produits sont déjà disponibles au comptoir soit ils nous seront livrés très bientôt. Dans tous les cas, notre équipe de concierges vous livrera directement en chambre dès réception et vous contactera pour les prestations bien-être.
Notre sélection Braderie Voir tout


Vos produits préférés

Vos coups de cœur Cadeaux de naissance bébé et maman Voir tout

La conciergerie happytal propose pour chacun de ses hôpitaux et cliniques partenaires, un catalogue de produits et services pour les patients et le personnel de santé. CHRU Tours - Bretonneau situé à Tours a mis en place cette conciergerie en ligne pour adoucir le séjour des patients, grâce à des milliers de produits comme l’alimentaire (chocolat, déjeuner, petit-déjeuner, gâteaux) ou du divertissement (lecture, musique, jeux de société) ou encore de la mode, des fleurs, etc. Il y a aussi tous les produits de maternité nécessaires aux mamans et à leurs bébés ainsi que des cadeaux pour célébrer les naissances. Nos produits et services sont proposés pour tous les âges, des enfants en Pédiatrie jusqu’aux séniors en Gériatrie et répondent à tous les besoins selon le service d’hospitalisation (diabétologie, chirurgie, oncologie, …). La conciergerie en ligne happytal est la première conciergerie dédiée au monde hospitalier, pour les patients, les proches mais aussi le personnel de santé.
Nos concierges qui sont sur place, réceptionnent les colis et les livrent directement en chambre, pour plus de confort. Ces personnes sont aussi là pour écouter, accompagner et apporter du sourire quand il y en a besoin !
Advertisement
Introduction
Patients, materials, and methods, acknowledgments, rituximab (anti-cd20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Philippe Colombat , Gilles Salles , Nicole Brousse , Pitahey Eftekhari , Piere Soubeyran , Vincent Delwail , Eric Deconinck , Corinne Haı̈oun , Charles Foussard , Catherine Sebban , Astasia Stamatoullas , Noël Milpied , François Boué , Bruno Taillan , Pierre Lederlin , Albert Najman , Catherine Thièblemont , François Montestruc , Anne Mathieu-Boué , Aziz Benzohra , Philippe Solal-Céligny; Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood 2001; 97 (1): 101–106. doi: https://doi.org/10.1182/blood.V97.1.101
Download citation file:
- Ris (Zotero)
- Reference Manager
The clinical activity of rituximab, a chimeric monoclonal antibody which binds to the CD20 antigen, was evaluated as a single first-line therapy for patients with follicular non-Hodgkin lymphoma (NHL). Fifty patients with follicular CD20 + NHL and a low tumor burden were analyzed for clinical and molecular responses. They received 4 weekly infusions of rituximab at a dose of 375 mg/m 2 . The response rate a month after treatment (day 50) was 36 of 49 (73%), with 10 patients in complete remission, 3 patients in complete remission/unconfirmed, and 23 patients in partial remission. Ten patients had stable disease, and the disease progressed in 3 patients. One of 13 (8%) patients in complete remission, 9 of 23 (39%) patients in partial remission, and 5 of 10 (50%) patients with stable disease exhibited disease progression during the first year. Within the study population, 32 patients were initially informative for polymerase chain reaction (PCR) data on bcl-2–J H rearrangement. On day 50, 17 of 30 patients (57%) were negative for bcl-2–J H rearrangement in peripheral blood, and 9 of 29 (31%) were negative in bone marrow; a significant association was observed between molecular and clinical responses ( P < .0001). At month 12, 16 of 26 patients (62%) were PCR negative in peripheral blood. These results indicate that early molecular responses can be sustained for up to 12 months and that this response is highly correlated with progression-free survival. Rituximab has a high clinical activity and a low toxicity and induces a high complete molecular response rate in patients with follicular lymphoma and a low tumor burden.
The optimal treatment of advanced-stage follicular non-Hodgkin lymphoma (NHL) is yet to be determined. In patients with a low tumor burden and without adverse prognostic factors, retrospective analysis 1 and prospective studies 2 , 3 have shown that postponing treatment until progression has no negative influence on survival. Nevertheless, almost all patients will experience disease progression and ultimately die of their disease. Thus, new treatment approaches for this subgroup of patients are needed and must meet several requirements—high efficacy, low morbidity, minimal influence on the sensitivity of lymphoma cells to subsequent treatments, and, especially, no induction of chemoresistance.
Recent studies have been devoted to antibody-mediated therapy. Treatments with unmodified monoclonal antibodies (mAbs) have included patient-specific mAbs, 4 , 5 anti-CD20, 6 anti-CD19 and CD22 Abs coupled with immunotoxins, 7-9 and anti-CD20 mAbs radiolabeled with iodohippurate sodium I 131 or yttrium Y 90 in low doses 10 , 11 and in high doses with stem cell support. 12 , 13 The chimeric human–mouse anti-CD20 mAb rituximab is a human IgG1κ antibody, with mouse variable regions isolated from a murine anti-CD20 mAb, IDEC-2B8. 14 In vitro studies showed that this chimeric antibody is able to lyse CD20 + cells using human-complement or antibody-dependent cell-mediated cytotoxicity (ADCC) 14 and that it induces apoptosis in the presence of either goat antimouse IgG or Fc receptor-expressing cells. 15
Several trials 16-18 have shown that rituximab as single therapy has a significant clinical activity in pretreated patients with follicular lymphoma. 16-18 Recently, results of a large phase II trial were reported. 19 One hundred sixty-six patients with relapsed low-grade or follicular lymphoma were given 4 weekly doses of 375 mg/m 2 rituximab as outpatients; 48% of patients responded, and the median time to progression was 13 months among responders. Adverse events were mostly moderate and were limited to infusion-related events, especially during the first infusion. Overall, severe reactions to rituximab are rare and are observed mainly in patients with bulky tumors, 20 leukemic involvement, 21 or both.
However, the clinical activity of rituximab alone as a first-line therapy in patients with low-grade NHL has been evaluated only in a limited number of patients with various histologic subtypes of low-grade NHL. 22 , 23 This report summarizes results of a multi-institutional trial of a 4-dose course of rituximab in previously untreated patients with follicular lymphoma with a low tumor burden. The objectives of the study were: (1) to assess clinical activity; (2) to monitor molecular responses in blood and bone marrow and to correlate clinical and molecular responses; and (3) to assess toxicity in patients without bulky tumor.
Patients were eligible for inclusion in this study if they had follicular CD20 + NHL according to the REAL classification, 24 whatever the cell type. All slides of pathologic specimens were reviewed by one hemato-pathologist. Patients were required to have histologic diagnosis of NHL established less than 5 months before treatment with rituximab and no prior therapy, including steroids or radiation treatment. They were 18 to 75 years of age. Disease stage had to be II to IV according to Ann-Arbor classification, and the presence of at least one measurable site was required. Bone marrow involvement was not considered measurable. The study protocol was approved by the institutional ethics committee, and informed written consent was obtained from each patient before therapy.
Patients were required to have a low tumor burden according to the GELF criteria 3 —that is, no nodal or extranodal involvement of more than 7 cm, no B symptoms, no significant splenomegaly, no pleural effusion, no complications such as ascites or organ compression, and normal serum lactate dehydrogenase and β 2 -microglobulin levels. Patients had to have good performance status (0 or 1 on the World Health Organization scale) and adequate hematologic, renal, and hepatic functions. They were excluded if they had human immunodeficiency virus, hepatitis B surface antigen, hepatitis B core antigen, hepatitis C virus antibodies, or if they were pregnant.
Rituximab (Mabthera; Produits Roche, Neuilly, France) was given at a dose of 375 mg/m 2 by intravenous infusion for a total of 4 dosages (days 1, 8, 15, 22) on an outpatient basis. The drug was reconstituted in normal saline to a concentration of 1 mg/mL. Rituximab was administered intravenously at an initial rate of 50 mg/h. If no toxicity was observed during the first hour, the infusion rate was increased to 50 mg/h every 30 minutes, to a maximum rate of 300 mg/h. The infusion was interrupted if the patient contracted hypotension, edema, fever, rigors, dyspnea, or any other serious adverse effect. If that occurred, infusion was resumed at half the previous rate after resolution of the event. Oral premedication with acetaminophen and diphenhydramine hydrochloride was recommended. No steroids were given.
Clinical monitoring
To assess all sites of disease involvement, baseline evaluation included clinical documentation, radiography of the chest, computed tomography (CT) of the chest, abdomen, and pelvis, and unilateral bone marrow biopsy. Laboratory testing included routine hematology, serum chemistries, serum immunoglobulin levels, lactate dehydrogenase, and β 2 -microglobulin assays in blood and urinalysis. Monitoring included hematology and serum chemistry evaluations before each treatment and full tumor restaging 28 days after the end of treatment, 1 month after that, every 3 months for 1 year, and then every 6 months for 2 years.
Molecular analysis of bcl-2–J H gene rearrangement
All samples were centralized in a single laboratory, and DNA was extracted using standard procedures and the usual precautions to avoid cross-contamination. For each assay at diagnosis, 1 μg DNA was amplified using MBR - (TATGGTGGTTTGACCTTTAG) or mcr - (CGTGCTGGTACCACTCCTG′) specific oligonucleotides, together with J H consensus (ACCTGAGGAGACGGTGACCAGGGT) oligonucleotide and AmpliTaq Gold polymerase (Perkin Elmer). Each reaction included 10 minutes at 95°C, then 50 cycles—each comprising 3 steps at 94°C for 30", 56°C (MBR) or 58°C (mcr) for 30", and 72°C for 30"—followed by 9′ at 72°C. Amplified products were visualized on agar gel stained with ethidium bromide; under these conditions, the sensitivity of detection did not exceed 10 −3 For follow-up samples, nested polymerase chain reaction (PCR) analysis was performed using MBR (CAGCCTTGAAACATTGATGG) or mcr (CGTGCTGGTACCACTCCTG) with J H - (ACCTGAGGAGACGGTGACC) specific primers for the first round (30 cycles for MBR, 25 cycles for mcr), then a reamplification of 4% of the reaction product with internal MBR (CTATGGTGGTTTGACCTTTAGAG) or mcr (GGACCTTCCTTGGTGTGTTG) and J H (ACCAGGGTCCCTTGGCCCCAG) oligonucleotides (30 cycles each) under the same conditions as above, except that each PCR step lasted 1 minute. The sensitivity of this assay was routinely greater than or equal to 10 −4 . All samples at the time of diagnosis and during follow-up were tested twice, and all negative results were controlled with another PCR assay using tumor necrosis factor gene primers 25 to ensure DNA integrity.
The primary efficacy endpoint was the objective response rate—that is, the proportion of patients achieving either complete remission (CR) or partial response (PR) according to the criteria recently reported by Cheson et al. 26 Complete response required the resolution of all symptoms and the disappearance of all detectable clinical and radiologic lesions (greatest transverse diameter, less than 1.5 cm), including bone marrow cleansing, for at least 28 days. Complete remission/unconfirmed (CRu) was defined by either a residual lymph node mass larger than 1.5 cm at the greatest transverse diameter, with the sum of the products of perpendicular diameters regressing by more than 75%, or an indeterminate bone marrow result (increased number or size of aggregates without atypical cytologic or architectural features). PR required a decrease greater than or equal to 50% of the sum of the products of perpendicular diameters of the 6 largest dominant nodes or nodal masses without any evidence of disease progression for at least 28 days. Progressive disease was defined as any occurrence of a new lesion during or at the end of therapy or a 50% increase in the size of any previously identified lesion. Stable disease was defined as no change of the target lesions or a change not corresponding to PR or progressive disease. An independent panel of radiologists reviewed all CT scans of all the included patients.
Clinical response was evaluated on days 50 and 78, and maximal response at either of these stagings (if confirmed on day 180 and if the maximal response was observed on day 78) was taken into account for this report. Comparison of clinical response by individual prognostic variables was performed using logistic regression.
All patients were evaluated for progression at 12 months according to the Cheson et al 26 criteria. Patients in CR or CRu with bone marrow (BM) cleansing on day 50 and BM showing again some involvement at 12 months were considered to have progressive disease. Patients in PR with negative BM biopsy findings on day 50 and minimal or moderate involvement at 12 months were not considered in progression because the latter could be related to technical aspects (sample size). An independent panel of lymphoma specialists reviewed all responses. Comparison of clinical responses at 12 months with the day 50 results of PCR analysis was performed using the Fisher exact test.
The secondary endpoints were PCR outcome and progression-free survival. Progression-free survival was calculated according to the method of Kaplan and Meier 27 and was measured from the start of treatment until progression/relapse or death. Comparison of the survival functions by results of PCR analysis on day 50 was performed using the log-rank test.
Patient characteristics
Fifty patients were enrolled in 16 centers from October 1997 to August 1998. One patient was excluded after histologic review. Thus, 49 patients were evaluable for response and 50 for tolerance.
There were 26 men and 24 women. The median age was 52 years (range, 32 to 75 years). Twenty-two patients had grade I, 24 had grade II, and 3 had grade III follicular NHL. Four patients had Ann Arbor stage II, 11 had Ann Arbor stage III, and 35 had Ann Arbor stage IV disease. Sixty-six percent of patients had bone marrow involvement, and 82% of the patients had at least 2 measurable sites (Table 1 ).
Patient characteristics (n = 50)
Clinical response rate
The response rate (RR) was 73% (36 of 49) with 10 patients (20%) in CR, 3 patients (6%) in CRu, and 23 patients (47%) in PR. Ten patients (20%) had stable disease, whereas 3 patients (6%) experienced disease progression during treatment. Lung carcinoma was diagnosed in one patient 10 months after treatment, but it appeared to have existed at the time of NHL diagnosis. The lung radiologic abnormality was considered lymphoma involvement. Hodgkin disease developed in another patient 11 months after treatment.
Sex, stage, bone marrow involvement, number of extranodal sites, and detectable bcl-2 gene rearrangement by PCR in peripheral blood at diagnosis were analyzed to define individual prognostic variables for clinical response. No factor was found of prognostic value.
Of the 36 patients who were responders on day 78, 10 patients progressed during the first year of follow-up (1 CR and 9 PR); 5 of the 10 patients with stable disease were also progressing at 12 months (Figures 1 , 2 ). Two progressions to Hodgkin lymphoma and diffuse large-cell lymphoma were observed. Of note, we observed that 7 of 23 patients in PR on day 78 were in CR or CRu at 12 months and that 3 of 10 patients with stable disease on day 78 were in PR at 12 months. Thus, if we consider the response rate at any staging during the first year of follow-up after rituximab treatment, 20 of 49 patients (41%) reached CR/CRu, and 19 of 49 patients (39%) reached PR, for an overall response rate of 39 of 49 patients (80%).

Progression-free survival of all patients evaluable for response (n = 49).

Relapse-free survival of responders (n = 36).
Results of the PCR analysis of bcl-2–J H rearrangement
Of the 49 patients with follicular lymphoma included in the study, 48 were evaluated using PCR analysis before treatment. The bcl-2–J H rearrangement was detected in peripheral blood in 32 of 48 patients tested, in 29 of 45 of those patients tested in bone marrow, and in 11 of 17 patients tested in lymph node. All patients who were positive in peripheral blood were also positive in lymph node or bone marrow, and this 32-patient (67%) total was therefore considered informative for further follow-up, 28 with a rearrangement in the MBR region and 4 in the mcr region.
On day 50, after the start of rituximab treatment, 30 patients were evaluated in peripheral blood and 17 of them (57%) were negative for bcl-2–J H rearrangement (Table 2 ). Among the latter, 9 were also PCR negative in bone marrow, whereas 7 remained PCR positive in bone marrow (one patient did not have bone marrow evaluation). Therefore, 9 of 30 (30%) of the evaluable patients had a clearance of the molecular markers in both blood and bone marrow. When molecular response was compared with clinical response assessed on days 50 and 78, a significant association ( P < .0001) was observed between molecular and clinical responses: 10 of the 17 patients who were PCR negative in peripheral blood were in CR/CRu, whereas none of the 13 patients PCR positive in peripheral blood had CR (Table 2 ).
Results of the bcl-2–J H PCR analysis on day 50 and clinical outcome (n = 30)
Only 30 of 32 patients with bcl-2–J H rearrangement at diagnosis were evaluated on day 50.
2-tailed Fisher exact test for comparison of PCR analysis on day 50 and clinical outcome at 12 months ( P < .0001).
All patients who were PCR positive in peripheral blood were also PCR positive in bone marrow, except 2 untested in bone marrow.
2-tailed Fisher exact test for comparison of PCR analysis on day 50 and clinical outcome on day 78 ( P < .0001).
The data from follow-up samples obtained at 6 months in peripheral blood and at 12 months in peripheral blood and bone marrow showed that the overall proportions of patients who were PCR negative in peripheral blood were comparable at the 3 time points—17 of 30 (57%), 18 of 26 (69%), and 16 of 26 (62%), respectively—indicating that the PCR response was maintained over that period. The kinetics and duration of the molecular response were assessed by comparing the results obtained from patients on day 50 and at month 12 for whom serial samples were obtained and analyzed. Interestingly, 6 of 6 evaluable patients who were PCR negative in peripheral blood and bone marrow on day 50 remained PCR negative in both sites at 12 months. Of 7 patients with discordant results in peripheral blood and bone marrow, 4 became PCR negative in both sites, whereas 3 reverted to PCR positivity. Finally, 2 of 11 patients who were PCR positive in peripheral blood and bone marrow on day 50 became PCR negative at 12 months, whereas the 9 others remained PCR positive in blood, marrow (2 with negative PB), or both (Table 3 ). Interestingly, only 1 of 17 patients (6%) with negative PCR results in peripheral blood on day 50 had disease progression during the first year compared to 8 of 13 patients (62%) with PCR positive results (Table 2 ). Overall, these results indicate that early molecular responses in blood and bone marrow after rituximab treatment can be sustained for up to 12 months and that some patients may have delayed molecular response. Moreover, progression-free survival curves showed that early molecular response is associated with a lower rate of disease progression during the first year of follow-up ( P < .005) (Figure 3 ).
Kinetics and duration of the molecular response from day 50 until 12 months

Progression-free survival according to the results of bcl-2 PCR analysis in blood performed 1 month after treatment of 30 patients with initial bcl-2–J H rearrangement.
Adverse events
All patients received the 4 weekly infusions at full dose. The most common adverse events thought to be related to rituximab infusion are listed in Table 4 . The most frequent events were grade 1-2 fever, headache, asthenia, pain, rash, laryngitis, rhinitis, paresthesia, hypotension, and nausea. Two cases of grade 3-4 hypotension and hypertension resolved after management according to the protocol procedures. No hematologic toxicity was observed, and only one minor infection developed.
Incidence of adverse events per patient (n = 50)
We report a trial evaluating the clinical efficacy and safety of single-agent rituximab therapy as first-line therapy of follicular NHL with low tumor burden. In addition, patients were evaluated for molecular response in blood and bone marrow. Previous experience with 4 weekly courses of rituximab as single first-line therapy has been reported in refractory or relapsing low-grade lymphoma. 16-19 A pivotal multicenter trial 19 was performed in 166 patients with recurrent or refractory low-grade, mostly follicular, B-cell lymphoma. The overall response rate was 48% (6% CR, 42% PR), confirming the phase I-II data. The efficacy of rituximab had also been shown in an 8-week infusion program 28 and as retreatment. 29
We have treated 49 patients with follicular NHL and a low tumor burden; the response rate was 73%—CR, 20%; CRu, 6%; and PR, 47%. This rate is similar to the response rate to other agents—such as an alkylating agent or interferon alfa—in a similar subgroup of patients with follicular NHL. 3 We confirmed also that a delayed response could be observed after rituximab therapy. Ten patients had clinical responses between day 78 and month 12, so that the overall response rate was 79%. In the current study, no clinical or biologic parameters were found to be associated with the clinical response after rituximab treatment. This could be related to the low number of patients, but it is likely to reflect the homogeneity of the population, which comprised only patients with follicular lymphoma with a low tumor burden. Additional studies are warranted to identify patients who may not respond to rituximab.
The possibility of clearing the residual disease evaluated by PCR technique appears particularly interesting. On day 50, of 30 patients initially positive for bcl-2–J H rearrangement in peripheral blood, 17 were negative after treatment and 9 were also negative in bone marrow. Two other patients had a delayed response in peripheral blood. Serial PCR analysis after treatment showed that PCR response persisted over time. At 12 months, 12 of 26 patients remained PCR negative in blood and bone marrow. Conversely, a few patients (3 of 17) who became PCR negative in blood after treatment did not reach clinical response.
These results appear comparable to those reported in patients with progressive or relapsed disease. McLaughlin et al 19 and, more recently, Gupta et al 30 indicated reversion to negative status in blood and bone marrow of relapsed patients after rituximab therapy. A clear association was observed between PCR negativity and clinical response.
The significance of molecular remission in follicular lymphoma remains unclear. Recently, Lopez-Guillermo et al 31 tested, during and after treatment, the peripheral blood and, when possible, the bone marrow of 194 patients exhibiting either MBR or mcr bcl-2 rearrangement at diagnosis. Patients who achieved molecular response during the first year of treatment had a significantly longer failure-free survival than those who did not (4-year progression-free survival, 76% versus 38%). Similar prognostic value of bcl-2 negativity has been reported after autologous bone marrow transplantation. Gribben et al 32 have indicated that after autologous bone marrow transplantation, patients with negative blood bcl-2 PCR findings have significantly lower relapse rates and better progression-free survival rates than patients who remain bcl-2 positive. On the other hand, some patients with localized follicular NHL remain PCR positive during several years without clinical relapse. 33
In our group of patients, PCR negativity also appeared to be associated with a good prognosis because only one patient with PCR negativity has had a relapse during the first year (whereas 8 of 13 patients who were PCR positive after treatment have had relapses). Nevertheless, the clinical significance of molecular remission after rituximab therapy remains unknown. It may reflect either a true molecular response in all disease sites or a predominant effect of rituximab on blood and bone marrow involvements. Of note, we observed that most patients remained PCR negative after the usual delay of the reappearance of normal B-lymphocytes in blood and bone marrow that occurs 6 to 9 months after treatment with rituximab. 19
Importantly, these results were obtained without the toxicity of conventional chemotherapy. The adverse events consisted of grade 1 or 2 symptoms such as fever, headache, nausea, hypotension, rhinitis, angioedema, rash, pain, or pruritus. Only 2 cases of grade 3 toxicity, manifested as hypotension or hypertension, were observed during the first infusion. As described in previous trials, the number and the severity of adverse events decreased with subsequent infusions. The small number of adverse events could be explained by the low tumor burden of the patients included in our study. 20 We did not observe any appearance of severe infusion-related symptom complex recently described. 21
Two other studies of rituximab used as first-line treatment of low-grade NHL have been reported recently. Hainsworth et al 22 reported a 52% response rate among 25 patients with follicular lymphoma. Gutheil et al 23 observed 8 responses among 14 patients with follicular lymphoma. Molecular results were not reported in any of these studies.
In conclusion, these results demonstrate that rituximab has high clinical activity in previously untreated patients with follicular NHL. Response duration has yet to be determined. In addition, rituximab as a single agent induces a molecular response rate that has not been reported with any other cytotoxic agent. These responses have to be confirmed with larger phase III trials in which rituximab will be compared with other therapeutic approaches. Many additional issues about this agent remain to be explored: combination with chemotherapy, 34 with growth factors such as G-CSF 35 or GM-CSF which could increase ADCC, or with an immunotherapy such as interferon alfa. 36 Whether repeated treatments with rituximab of responders after recovery of CD20 + cells may delay clinical relapse remains to be investigated. This is being tested in another trial. 22
We thank Carole Charlot and Marie-Claude Chamard for expert technical assistance. We also thank J. N. Munck, F. Morschauser, B. Grosbois, and A. Delmer for clinical reviews; Y. Menu and A. Scherrer for radiologic reviews; and J. M. Goehrs (Medical Department, Produits Roche).
Supported by Produits Roche, Neuilly-sur-Seine, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement ” in accordance with 18 U.S.C. section 1734.
Author notes
Ph. Solal-Celigny, Centre Jean Bernard, 9 rue Beauverger, 72000 Le Mans, France; e-mail: [email protected] .
This feature is available to Subscribers Only
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- First edition
- Collections
- Submit to Blood
- About Blood
- Subscriptions
- Public Access
- Permissions
- Blood Classifieds
- Advertising in Blood
- Terms and Conditions
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

A Study to Evaluate Subcutaneous Daratumumab in Combination With Standard Multiple Myeloma Treatment Regimens
- Study Details
- Tabular View
- Study Results

Inclusion Criteria:
- Multiple myeloma diagnosed according to the International Myeloma Working Group (IMWG) diagnostic criteria
Measurable, secretory disease as defined by any of the following:
- Serum monoclonal paraprotein (M-protein) level greater than or equal to (>=) 1.0 gram per deciliter (g/dL); or
- Urine M-protein level >= 200 milligram per 24 hours (mg/24 hours); or
- Light chain multiple myeloma (MM), for participants without measurable disease in the serum or urine: serum Immunoglobulin (Ig) free light chain (FLC) >= 10 mg/dL and abnormal FLC ratio
Meets one of the sets of the following criteria:
- For Daratumumab + bortezomib + lenalidomide + dexamethasone (D-VRd) and Daratumumab + bortezomib + melphalan + prednisone + dexamethasone (D-VMP) regimen: newly diagnosed myeloma
- For Daratumumab + lenalidomide + dexamethasone (D-Rd) and Daratumumab + Carfilzomib + Dexamethasone (D-Kd) regimen: relapsed or refractory disease
- D-Kd cohort: Participants must have received only 1 prior line of therapy for MM which included at least 2 consecutive cycles of lenalidomide therapy
- Eastern Cooperative Oncology Group (ECOG) Performance Status grade of 0, 1, or 2
- During the study, during dose interruptions, and for 3 months after receiving the last dose of any component of the study treatment, a female participant must agree not to donate eggs (ova, oocytes) and male participants of reproductive potential must not donate semen or sperm during the study, during dose interruptions, or for 3 months after the last dose of any study drug
Exclusion Criteria:
- History of malignancy (other than MM) unless all treatment of that malignancy was completed at least 2 years before consent and the participant has no evidence of disease further exceptions are squamous and basal cell carcinomas of the skin and carcinoma in situ of the cervix, or breast, or other non-invasive lesion, that in the opinion of the investigator, with concurrence with the sponsor's medical monitor, is considered cured with minimal risk of recurrence within 3 years
- Exhibits clinical signs of meningeal involvement of MM
- Either of the following: a) Chronic obstructive pulmonary disease with a forced expiratory volume in 1 second (FEV1) is less than (<) 50 percentage (%) of predicted normal b) Moderate or severe persistent asthma, or a history of asthma within the last 2 years, or currently has uncontrolled asthma of any classification c) For D-Kd cohort: Known infiltrative pulmonary disease or known pulmonary hypertension
- Any of the following: a) Known to be seropositive for human immunodeficiency virus; b) Seropositive for hepatitis B (defined by a positive test for hepatitis B surface antigen [HBsAg]). Participants with resolved infection (participants who are HBsAg negative but positive for antibodies to hepatitis B core antigen [Anti-HBc] and/or antibodies to hepatitis B surface antigen [Anti-HBs]) must be screened using real-time polymerase chain reaction (PCR) measurement of hepatitis B virus (HBV) DNA levels. Those who are polymerase chain reaction (PCR) positive will be excluded
- Known to be seropositive for hepatitis C (Anti-HCV antibody positive or HCV-RNA quantitation positive) except in the setting of a sustained virologic response [SVR], defined as aviremia at least 12 weeks after completion of antiviral therapy
- For D-Kd cohort only: Transthoracic echocardiogram showing left ventricular ejection fraction (LVEF) <40%; uncontrolled hypertension, defined as an average systolic blood pressure greater than (>)159 millimeters of mercury (mmHg) or diastolic >99 mmHg despite optimal treatment

- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
Diagnosis of invasive pulmonary aspergillosis: updates and recommendations
Affiliations.
- 1 Service de parasitologie-mycologie-médecine tropicale, pôle de biologie médicale, hôpital Bretonneau, CHU de Tours, bâtiment B2A, 1(er) étage, 2, boulevard Tonnellé, 37044 Tours cedex 9, France; CEPR-UMR Inserm U1100/EA 6305, faculté de médecine, université François-Rabelais, 37032 Tours cedex 1, France. Electronic address: [email protected].
- 2 Service de parasitologie-mycologie-médecine tropicale, pôle de biologie médicale, hôpital Bretonneau, CHU de Tours, bâtiment B2A, 1(er) étage, 2, boulevard Tonnellé, 37044 Tours cedex 9, France.
- 3 Service de parasitologie-mycologie-médecine tropicale, pôle de biologie médicale, hôpital Bretonneau, CHU de Tours, bâtiment B2A, 1(er) étage, 2, boulevard Tonnellé, 37044 Tours cedex 9, France; CEPR-UMR Inserm U1100/EA 6305, faculté de médecine, université François-Rabelais, 37032 Tours cedex 1, France.
- PMID: 24548415
- DOI: 10.1016/j.medmal.2013.11.006
Invasive pulmonary aspergillosis is an opportunistic mycosis, difficult to diagnose, due to the environmental fungi of the genus Aspergillus. The diagnostic tools, even if more are available, are still limited in number and effectiveness. The current recommendations issued by the EORTC/MSG (European Organization of Research and Treatment of Cancer/Mycoses Study Group) and the ECIL (European Conference for Infection in Leukemia) suggest collecting epidemiological, radio-clinical, and biological data to support the diagnosis of aspergillosis with a strong presumption. Thus, medical imaging and serum galactomannan antigen currently constitute the basis of the screening approach, although they both have some limitations in specificity. (1→3)-β-D-glucans are pan-fungal serum markers with a very good negative predictive value. Real-time PCR lacks standardization, and fungal culture from respiratory specimens is sometimes not sensitive enough. Histology allows proving the diagnosis of aspergillosis, but biopsy is not always possible in immunodepressed patients. We present the various arguments for the diagnosis of invasive aspergillosis, with a particular emphasis on recent exploration techniques.
Keywords: Aspergillose invasive; Invasive aspergillosis; Recommandations; Recommendations.
Copyright © 2013 Elsevier Masson SAS. All rights reserved.
Publication types
- Antibodies, Fungal / blood
- Antigens, Fungal / blood
- Aspergillus / growth & development
- Aspergillus / immunology
- Aspergillus / isolation & purification
- DNA, Fungal / analysis
- Diagnostic Imaging
- Early Diagnosis
- Galactose / analogs & derivatives
- Immunocompromised Host
- Invasive Pulmonary Aspergillosis / blood
- Invasive Pulmonary Aspergillosis / diagnosis*
- Invasive Pulmonary Aspergillosis / epidemiology
- Invasive Pulmonary Aspergillosis / pathology
- Mannans / blood
- Practice Guidelines as Topic
- Predictive Value of Tests
- Real-Time Polymerase Chain Reaction
- Sensitivity and Specificity
- Tomography, X-Ray Computed
- beta-Glucans / blood
- Antibodies, Fungal
- Antigens, Fungal
- DNA, Fungal
- beta-Glucans
- galactomannan
Pruvo – Saving Money After Booking
Covid-19 pcr tests in moscow – russia.
If you are traveling to Moscow and must perform a Covid-19 PCR test before your flight, below is a list of Covid-19 PCR test centers in Moscow, Russia.
A little tip for big savings: Download Pruvo´s app, which helps you lower the price of the hotels you have already booked by alerting you when price drops occur after booking the hotel. This way you can save hundreds of dollars effortlessly.
Plan your trip easily with Wiki for travel
Centers for molecular detection of Coronavirus (SARS-CoV-2), more commonly referred to as PCR tests, Antigen Tests, or Rapid Lateral Flow tests, can be found on this page. PCR Tests, (Real-time polymerase chain reaction (RT–PCR) is a nuclear-derived technology for identifying the presence of specific genetic material in any disease, including viruses. It’s one of the most extensively utilized COVID-19 viral detection methods in laboratories. An Antigen test is a rapid diagnostic test suitable for point-of-care testing that directly detects the presence or absence of an antigen. Rapid lateral flow tests are required for UK travelers and involve rubbing a long cotton bud (swab) over your tonsils (or where they would have been) and inside your nose.
All Covid-19 PCR Test / Rapid Antigen Test / Rapid Lateral Flow test centers that appear in this list have been certified by the country´s state health department. If you need your Covid-19 diagnostic test prior to your flight, make sure to provide the exact name that appears in your passport (including middle name) and request a test certificate in English.
If you are on vacation or holiday and have symptoms such as fever, cough, tiredness, loss of taste, loss of smell, difficulty breathing, chills, sore throat, runny nose, headaches, chest discomfort, or diarrhea, it´s highly recommended that you conduct a Covid-19 test and auto-isolate till the results are back.
Prior to going for your PCR, Antigen, or Rapid Lateral Flow test, make sure to contact the laboratory and ask if you need to make an appointment or if they accept walk-ins. Some RT-PCR test laboratories offer expedited results (which may incur an additional fee). Not all Covid-19 diagnostic test centers are open on weekends, so plan accordingly and make sure to consult the lab prior to going for your Coronavirus test.
In case you found a mistake or want us to add more information about Covid-19 PCR Test / Rapid Antigen Test / Rapid Lateral Flow test , please contact us and we will be more than happy to share the additional information. It is important to mention that Pruvo has developed this database of Covid-19 PCR Test / Rapid Antigen Test / Rapid Lateral Flow test in order to help you save time and money when traveling abroad or back home.

- Standard et urgences
- --> --> --> Espace patient --> -->