You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Hepatitis A
- Section 5 - Hepatitis C
Hepatitis B
Cdc yellow book 2024.
Author(s): Aaron Harris
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Hepatitis B virus
High prevalence in Africa and the Western Pacific
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Ensure sterile medical and dental techniques
Use safe injection practices
Hepatitis B is a vaccine-preventable disease
DIAGNOSTIC SUPPORT
Hepatitis B virus (HBV) is a small, circular, partially double-stranded DNA virus in the family Hepadnaviridae .
HBV is transmitted by contact with contaminated blood, blood products, and other body fluids (e.g., semen). Travelers could be exposed to HBV through poor infection control during dental or medical procedures, receipt of blood products, injection drug use, tattooing or acupuncture, or unprotected sex.
HBV is a leading cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma worldwide. In 2015, an estimated 257 million people globally were living with chronic HBV infection; that year, HBV caused an estimated 887,000 deaths. HBV infections are likely underestimated, however, because accurate data are lacking from many countries ( Map 5-07 ).
Data demonstrating the specific risk to travelers are lacking; published reports of travelers acquiring hepatitis B are rare, however, and the risk for travelers who do not have high-risk behaviors or exposures is low. The risk for HBV infection might be higher in countries where the prevalence of chronic HBV infection is ≥2% (e.g., in the western Pacific and African regions); expatriates, missionaries, and long-term development workers in those regions might be at increased risk for HBV infection. All travelers should be aware of how HBV is transmitted and take measures to minimize their exposures.
Map 5-07 Worldwide prevalence of hepatitis B virus infection
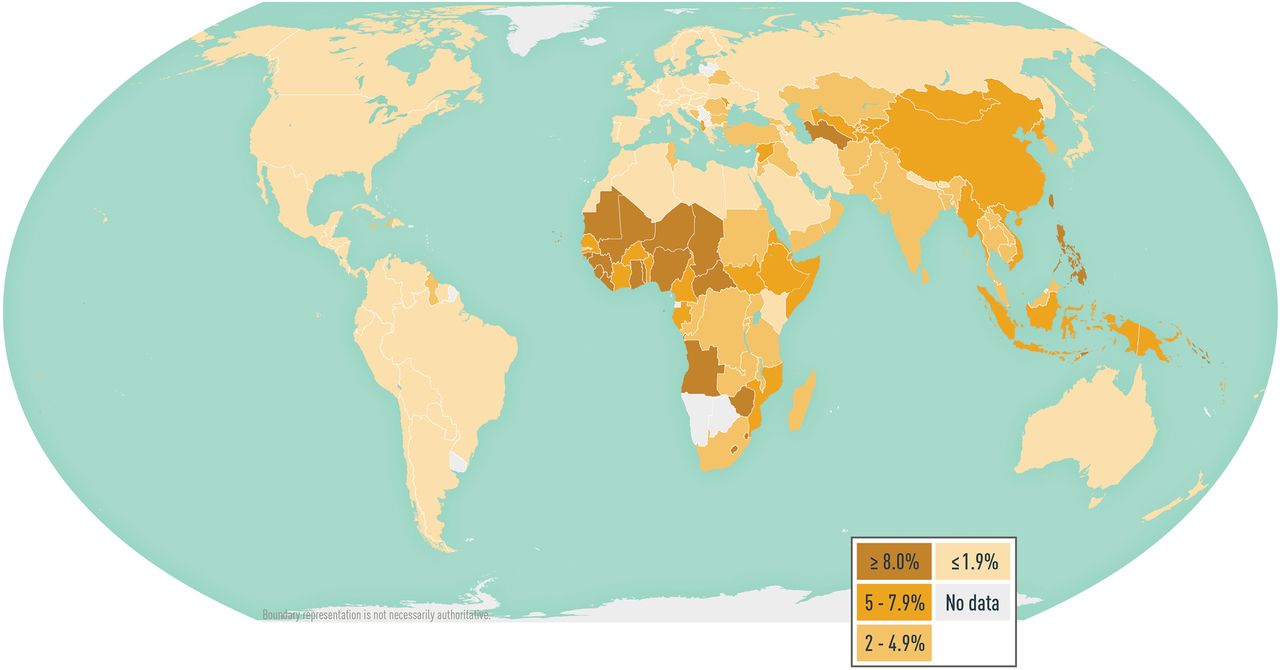
View Larger Figure
Disease data source: 2021 estimates of hepatitis B virus disease burden. CDA Foundation Polaris Observatory. Available from: https://cdafound.org/polaris-countries-distribution/ .
HBV infection primarily affects the liver. Typically, the incubation period for hepatitis B is 90 days (range 60–150 days). Newly acquired acute HBV infections only cause symptoms some of the time, and signs and symptoms vary by age. Most children <5 years of age and immunosuppressed adults are asymptomatic when newly infected, whereas 30%–50% of newly infected people aged ≥5 years have signs and symptoms. When present, typical signs and symptoms of acute infection include abdominal pain, anorexia, fatigue, fever, jaundice, joint pain, malaise, nausea and vomiting, light (clay-colored) stool, and dark urine. The overall case-fatality ratio of acute hepatitis B is ≈1%.
Some acute HBV infections resolve on their own, but some develop into chronic infection. The risk for acute hepatitis B to progress to chronic HBV infection depends on the age at the time of initial infection as follows: >90% of neonates and infants, 25%–50% of children aged 1–5 years, and <5% of older children and adults. Most people with chronic HBV infection are asymptomatic and have no evidence of liver disease. Fifteen percent to 40% of people with chronic HBV infection will, however, develop liver cirrhosis, hepatocellular carcinoma, or liver failure, and 25% of chronically infected people die prematurely from these complications. People infected with HBV are susceptible to infection with hepatitis D virus; coinfection increases the risk for fulminant hepatitis and rapidly progressive liver disease.
Hepatitis B is a nationally notifiable disease. The clinical diagnosis of acute HBV infection is based on signs or symptoms consistent with viral hepatitis and elevated hepatic transaminases and cannot be distinguished from other causes of acute hepatitis. Serologic markers specific for hepatitis B are necessary to diagnose HBV infection and for appropriate clinical management ( Table 5-11 ). These markers can differentiate between acute, resolving, and chronic infection.
See information on how to obtain hepatitis B diagnostic support from the Centers for Disease Control and Prevention (CDC) Infectious Diseases Laboratories, including contact information, which samples to send, and how to send samples. Select Hepatitis B Genotyping for research use only, and Hepatitis B Serology and Quantitative PCR if testing regulated by Clinical Laboratory Improvement Amendments is needed.
Table 5-11 Interpretation of serologic test results for hepatitis B virus infection 1
Abbreviations: HBsAg, hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen.
1 Adapted from Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804.
2 Hepatitis B-directed care includes a physical examination and laboratory evaluation for liver transaminase, hepatitis B virus DNA, and hepatitis B e antigen.
3 An anti-HBs titer of ≥10 mIU/mL correlates with protection only after a documented, complete hepatitis B vaccine series.
4 If false-positive results are suspected, repeat testing. If results are from past infection or passive transfer to infants born to HBsAg-positive mother, no specific action is needed. If results could indicate occult hepatitis B virus infection, inform patient of risks from future chemotherapy, immunosuppression, or hepatitis C virus infection antiviral therapy, and consider checking hepatitis B virus DNA.
No medications are available to treat acute HBV infection; treatment is supportive. Several antiviral medications are available for people with chronic HBV infection. People with chronic HBV infection should be under the care of a health professional and receive a thorough physical examination and laboratory testing to determine the need for antiviral therapy and ongoing monitoring for hepatocellular carcinoma and liver damage. See American Association for the Study of Liver Diseases (AASLD) practice guidelines for the treatment of chronic HBV infection .
Indications for Use
Administer Hepatitis B vaccine to all unvaccinated people traveling to areas with intermediate to high prevalence of chronic HBV infection, namely, countries with HBV surface antigen positivity prevalence ≥2% ( Map 5-07 ). See complete vaccination information and recommendations for the United States .
Administration
Several hepatitis B vaccines are available ( Table 5-12 ). Hepatitis B vaccines are administered either as a 2-dose series at 0 and 1 month (Heplisav-B [Dynavax Technologies Corporation]), or as a 3-dose series at 0, 1, and 6 months (Engerix-B [GlaxoSmithKline], Recombivax HB [Merck], PreHevbrio [VBI], and the combined hepatitis A and hepatitis B vaccine, Twinrix [GlaxoSmithKline]). Heplisav-B is licensed for a 2-dose schedule for adults aged ≥18 years; the second dose should be given ≥1 month after the first dose.
Engerix B and Recombivax HB have also been licensed for use according to alternative vaccination schedules. Engerix-B can be administered using a 4-dose schedule, with the first 3 doses given within 2 months and a booster at 12 months (doses at 0, 1, 2, and 12 months). Recombivax HB can be given using a 2-dose schedule for children aged 11–15 years. Twinrix can be used on an accelerated 4-dose schedule (0, 7, and 21–30 days, with a booster at 12 months) to promote long-term immunity.
Always consult the prescribing information when administering alternative schedules and formulations. Whenever feasible, use the same manufacturer’s vaccines to complete the patient’s vaccine series; do not, however, defer vaccination when the manufacturer of previously administered doses is unknown or when the vaccine from the same manufacturer is unavailable. The 2-dose Heplisav-B vaccine series only applies when both doses in the series consist of Heplisav-B. Series consisting of a combination of 1 dose of Heplisav-B and a vaccine from a different manufacturer should adhere to the 3-dose schedule.
Protection from the standard vaccination series is robust, and >95% of healthy people achieve immunity after completion of the vaccination series. Serologic testing and booster vaccination are not recommended before travel for immunocompetent adults who have been previously vaccinated. Consider postvaccination serologic testing, however, for people whose subsequent clinical management depends on knowledge of their immune status, including health care personnel and public safety workers at risk for blood or body fluid exposure; those who require (or might require) outpatient hemodialysis; HIV-infected people; sex partners of HBsAg-positive people; and other immunocompromised people (e.g., hematopoietic stem-cell transplant recipients or people receiving chemotherapy).
Table 5-12 Hepatitis B vaccines
Abbreviations: HBsAg, hepatitis B surface antigen; IM, intramuscular; ELU, ELISA units inactivated HAV; HAV, hepatitis A virus.
1 Consult the prescribing information for differences in dosing for patients receiving hemodialysis, and other immunocompromised patients.
Special Situations
The accelerated Twinrix vaccination schedule (0, 7, and 21–30 days, plus booster at 12 months) can be used for people traveling on short notice who face imminent HBV exposure or for emergency responders to disaster areas. Alternatively, Heplisav-B can be used as a 2-dose series at 0 and 4 weeks to protect against hepatitis B alone. Ideally, vaccination with Heplisav-B should begin ≥1 month before travel so the full vaccine series can be completed before departure. When using vaccines other than Heplisav-B, begin vaccination ≥6 months before scheduled travel. Because some protection is provided by 1 or 2 doses, initiate the vaccine series, if indicated, even if the series cannot be completed before departure. Vaccines will not confer optimal protection, however, until after the series is completed; advise travelers to complete the vaccine series upon return.
Safety & Adverse Reactions
Safe hepatitis B vaccines are available for people of all ages, and serious adverse reactions are rare. The most common adverse reactions are soreness at the injection site (3%–29%) and low-grade fever (temperature >99.9°F [37.7°C]; 1%–6%). Hepatitis B vaccines should not be administered to people with a history of hypersensitivity to any vaccine component, including yeast. The vaccine contains a noninfectious recombinant protein (hepatitis B surface antigen) and an adjuvant (either 1018 [small synthetic immunostimulatory cytidine- phosphate-guanosine oligodeoxynucleotide motif for Heplisav-B] or aluminum [for Engerix-B, Recombivax HB, PreHevbrio, Twinrix]).
HBV infection affecting a pregnant person can result in serious disease for the mother and chronic infection for the newborn. Limited data indicate no apparent increased risk for adverse events to the mother (or the developing fetus) after maternal vaccination with Engerix-B, Recombivax HB, or Twinrix; no data are available on the use of Heplisav-B or PreHevbrio in pregnant or breastfeeding people. Until safety data are available for Heplisav-B and PreHevbrio, therefore, pregnant or breastfeeding people needing hepatitis B vaccination should receive Engerix-B, Recombivax HB, or Twinrix.
Personal Protective Measures
As part of the pretravel education process, educate all travelers about exposure risks for hepatitis B and other bloodborne pathogens, including activities or procedures that involve piercing the skin or mucosa; receiving blood products; contaminated equipment used during cosmetic (e.g., tattooing or piercing), dental, or medical procedures; injection drug use; and unprotected sexual activity. Caution travelers against providers who use inadequately sterilized or disinfected equipment, who reuse contaminated equipment, or who do not use safe injection practices (e.g., reusing disposable needles and syringes).
HBV and other bloodborne pathogens can be transmitted if medical equipment is not sterile or if personnel do not follow proper infection-control procedures. Travelers should consider the health risks when receiving dental or medical care overseas; US embassy country-specific websites might have information on medical concerns. Advise travelers to strongly consider health risks before obtaining a body piercing or a tattoo when traveling to destinations where adequate sterilization or disinfection procedures might not be available or practiced.
CDC website: Hepatitis B
The following authors contributed to the previous version of this chapter: Aaron M. Harris
Bibliography
Abara WE, Qaseem A, Schillie S, McMahon BJ, Harris AM. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804.
CDA Foundation. Polaris observatory; 2019 [updated 2020 Mar 18]. Available from: https://cdafound.org/polaris .
Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15);455–8.
Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(RR-1):1–31.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99.
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Hepatitis B Vaccine
Key vaccine facts, who should have the vaccine, safety and side effects, ingredients, more information about the vaccine, related diseases, other vaccines in this category.
Page last updated Tuesday February 28, 2023
- GP practice services
- Health advice
- Health research
- Medical professionals
- Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skincare and conditions
Travel and vaccinations
Treatment and medication
Women's health
Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z

Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Hepatitis B vaccine
Peer reviewed by Dr Colin Tidy, MRCGP Last updated by Dr Hayley Willacy, FRCGP Last updated 15 Feb 2023
Meets Patient’s editorial guidelines
In this series: Travel vaccinations Hepatitis A vaccine Rabies vaccine Tick-borne encephalitis vaccine Typhoid vaccine Yellow fever vaccine
Anyone at risk of being infected with the hepatitis B virus should consider being immunised, including workers likely to come into contact with blood.
In this article :
What is hepatitis b, how is hepatitis b passed on, who needs the hepatitis b vaccination, the vaccination schedule, rapid vaccination schedule, are there any side-effects from hepatitis b vaccination, what if i come into contact with hepatitis b and am not immunised, who should not receive the hepatitis b vaccine.
The hepatitis B vaccine can also be used to prevent infection if, for example, you have had a needlestick injury and you are not immunised. Some people need blood tests to check if they are immune. See your practice nurse if you think you need this vaccine.
Continue reading below
Hepatitis B is an infection caused by the hepatitis B virus. The infection mainly affects the liver. However, if you are infected, the virus is present in body fluids such as blood, saliva, semen and vaginal fluid. In the UK it is estimated that about 1 person in 200 to 1,000 is infected with the hepatitis B virus. It varies widely depending on the part of the UK studied. It is much more common in other countries. It is most common in sub-Saharan Africa and East Asia.
If you are infected with the hepatitis B virus, the initial symptoms can range from no symptoms at all to a severe illness. After this initial phase, in a number of cases the virus remains in the body long-term. These people are called carriers. Some carriers do not have any symptoms but can still pass on the virus to other people.
About 1 in 4 carriers eventually develop a serious liver disease such as cirrhosis. In some cases liver cancer develops after a number of years. See the separate leaflet called Hepatitis B for more details of the disease .
If you are pregnant and are infected with the hepatitis B virus, you can pass it on to your baby as the baby is being born. Vaccinations for the baby can prevent this happening. So all pregnant women in the UK are offered testing for hepatitis B during each pregnancy. If the test is positive, the baby can be protected.
The hepatitis B virus is passed from person to person in one of these ways:
Blood-to-blood contact. For example, drug users sharing needles or other equipment which may be contaminated with infected blood. (Blood used for transfusion is now screened for hepatitis B virus.) Healthcare workers can be infected through accidental needlestick injuries.
Having unprotected sex with an infected person.
An infected mother passing it to her baby .
A human bite from an infected person. This is very rare.
Do I need vaccination?
If you're travelling abroad, you can find out if immunisation against hepatitis is recommended for any countries you are planning to visit from the NHS website Fitfortravel .
Some people travelling abroad are advised to have hepatitis B immunisation in certain circumstances - for instance, for repeated visits, long stays or if you are having medical treatment while abroad. You can find out if immunisation against hepatitis B is recommended for you if you're travelling to other countries by visiting the NHS website Fitfortravel.
Anyone who is at increased risk of being infected with the hepatitis B virus should consider being immunised. This includes:
Workers who are likely to come into contact with blood products, or are at increased risk of needlestick injuries, assault, etc. For example:
Medical laboratory workers.
Cleaners in healthcare settings.
Morticians.
Prison wardens.
Police officers and fire and rescue workers.
Staff at daycare or residential centres for people with learning disabilities where there is a risk of scratching or biting by residents.
People who inject street drugs. Also:
Their sexual partners.
The people they live with.
Their children.
People who change sexual partners frequently (in particular, sex workers).
People who live in close contact with someone infected with hepatitis B. (You cannot catch hepatitis B from touching people or normal social contact. However, close regular contacts are best immunised.)
People who regularly receive blood transfusions (for example, people with haemophilia).
People with certain kidney or liver diseases.
People who live in residential accommodation for those with learning difficulties. People who attend day centres for people with learning difficulties may also be offered vaccination.
Families adopting children from countries with a higher risk of hepatitis B, when the hepatitis B status of the child is unknown. (It is, however, advisable for the child to be tested for hepatitis B.)
Foster carers or if you live with foster children.
Prison inmates. Vaccination against hepatitis B is now recommended for all prisoners in the UK.
Travellers to countries where hepatitis B is common. In particular, those who place themselves at risk when abroad. The risk behaviour includes sexual activity, injecting drug use, undertaking relief work and/or participating in contact sports. Also, if you may need a medical or dental procedure in these countries and the procedure may not be done with sterile equipment.
Babies who are born to infected mothers.
You need three doses of the vaccine for full protection. The second dose is usually given one month after the first dose. The third dose is given five months after the second dose.
One to four months after the third dose you may need to have a blood test. You may need one if you are at risk of infection at work, especially as a healthcare or laboratory worker or if you have certain kidney diseases. Your doctor will be able to advise you if you need a blood test. This checks if your body has made proteins to protect you (antibodies) against the hepatitis B virus. If you have, you will not be able to get it (ie you are immune.)
You may then need a booster dose five years later. There is no need for a blood test before or after this.
The schedule is the same for the combined hepatitis A and B vaccine which is also available.
Routine immunisation schedule
From 2017 all infants born in the UK have been routinely offered a six-component vaccine (Hib-DTaP-hepatitis B-poliovirus). The new vaccine will replace the existing five-component vaccine to also give protection against hepatitis B virus (HBV) in addition to diphtheria, tetanus, pertussis, poliomyelitis, and Haemophilus influenzae type b disease. There will not be any change to the timing of the routine childhood vaccination schedule, with the hexavalent (6-component) vaccine replacing the vaccine previously given at 8, 12, and 16 weeks of age.
A schedule of giving three doses more quickly than usual may be used in some situations. That is, three doses with each dose a month apart. An even quicker schedule is also sometimes used. That is, the second dose given seven days after the first and the third dose given 21 days after the first.
These rapid schedules may be used if you are at very high risk of infection and need to be immune as soon as possible. For example, if you are soon to travel abroad, are new to prison or are sharing needles to inject drugs. However, a more rapid schedule may not be as effective for long-term immunity unless a fourth dose is given 12 months after the first dose. Your doctor will advise on the best schedule for your circumstances.
Side-effects are uncommon. Occasionally, some people develop soreness and redness at the injection site. Rarely, some people develop a mild high temperature (fever) and a flu-like illness for a few days after the injection.
Seek medical attention as soon as possible if you have been at risk from a possible source of infection and you are not immunised. For example, if you have a needlestick injury or have been bitten by someone who may have hepatitis B.
You should have an injection of immunoglobulin as soon as possible. This is an injection which contains antibodies against the virus. It gives short-term protection. You should also start a course of vaccination. The hepatitis B vaccine is very effective at preventing infection if given shortly after contact with hepatitis B.
Even if you have had the hepatitis B vaccine and are at risk of infection (for example, by having unprotected sex or sharing contaminated needles), you should ask your doctor for advice. You may be advised to have a booster vaccine or even an injection of immunoglobulin.
Babies who are born to infected mothers should have an injection of immunoglobulin as soon as possible after they are born. They should also be immunised. The first dose of vaccine is given within the first day after birth. This is followed by three further doses at 1 month, 2 months and 12 months of age. At 12 months, immunised babies have a blood test to check that the vaccine has worked.
If you have an illness causing a high temperature, it is best to postpone vaccination until after the illness.
You should not have a booster if you have had a severe reaction to this vaccine in the past.
The vaccine may be given if you are pregnant or breast-feeding and vaccination against hepatitis B is necessary.
Further reading and references
- Travel Health Pro ; National Travel Health Network and Centre (NaTHNaC)
- Immunisation against infectious disease - the Green Book (latest edition) ; UK Health Security Agency.
- Travellers' Health ; US Centers for Disease Control and Prevention
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 6 Jan 2028
15 feb 2023 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
- Skip to content
- Accessibility help
Immunizations - travel: References
Last revised in July 2023
- ABPI ( 2018 ) SPC for TicoVac 0.5 ml Suspension for injection in a prefilled syringe . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020a ) SPC for VAQTA Adult suspension for injection . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020b ) SPC for Menveo Group A,C,W135 and Y conjugate vaccine . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020c ) SPC for Nimenrix . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020d ) SPC for Boostrix-IPV . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020e ) SPC for Infanrix Hexa . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020f ) SPC for Infanrix . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020g ) SPC for REPEVAX . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2020h ) SPC for REVAXIS . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- ABPI ( 2021 ) SPC for AVAXIM suspension for injection in a pre-filled syringe . Electronic Medicines Compendium . Datapharm Communications Ltd. https://www.medicines.org.uk/emc [ Free Full-text ]
- BNF ( 2021 ) British National Formulary . BMJ Group and Pharmaceutical Press . https://bnf.nice.org.uk
- NaTHNaC ( 2021a ) Country Information . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021b ) Diseases in Brief: Hepatitis A . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021c ) Diseases in Brief: Meningococcal Disease . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021d ) Diseases in Brief: Polio . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021e ) Diseases in Brief: Typhoid Fever . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021f ) Diseases in Brief: Tetanus . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021g ) Diseases in Brief: Yellow Fever . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021h ) Diseases in Brief: Cholera . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021i ) Diseases in Brief: Hepatitis B . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021j ) Diseases in Brief: Japanese Encephalitis . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021k ) Diseases in Brief: Rabies . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021l ) Diseases in Brief: Tick-borne Encephelitis . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NaTHNaC ( 2021m ) Hajj and Umrah . National Travel Health Network and Centre . https://travelhealthpro.org.uk [ Free Full-text ]
- NHS ( 2020 ) Rabies Vaccination . NHS . https://www.nhs.uk [ Free Full-text ]
- PHE ( 2013a ) Hepatitis A: the green book, chapter 17 . Chapter 17 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2013b ) Poliomyelitis: the green book, chapter 26 . Chapter 26 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2013c ) Cholera: the green book, chapter 14. Chapter 14. Public Health England. . www.gov.uk [ Free Full-text ]
- PHE ( 2013d ) Tick-borne encephalitis: the green book, chapter 31. Chapter 31. Public Health England. . www.gov.uk [ Free Full-text ]
- PHE ( 2013e ) Consent: the green book, chapter 2 . Chapter 2 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2013f ) Immunisation procedures: the green book, chapter 4 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2013g ) Vaccine safety and adverse effects following immunisation: the green book, chapter 8 . Chapter 8 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2013h ) Rabies: the green book, chapter 27 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2016 ) Meningococcal: the green book, chapter 22 . Chapter 22 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2017a ) Contraindications and special considerations . Green Book, Chapter 6 . Public Health England . https://www.gov.uk [ Free Full-text ]
- PHE ( 2017b ) Hepatitis A vaccination in adults: temporary recommendations . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2018a ) The Green Book Chapter 20: Japanese Encephalitis . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2018b ) The Green Book Chapter 27: Rabies . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2019 ) The Green Book Chapter 18: Hepatitis B . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2020a ) The Green Book Chapter 35: Yellow Fever . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2020b ) The Green Book Chapter 33: Typhoid . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2020c ) The Green Book Chapter 30: Tetanus . Public Health England . http://www.gov.uk [ Free Full-text ]
- PHE ( 2021 ) Immunisation against infectious disease (The Green Book) . Public Health England . http://www.gov.uk [ Free Full-text ]
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Public health
- Health protection
- Infectious diseases
Hepatitis A: the green book, chapter 17
Hepatitis A immunisation information for public health professionals.
PDF , 136 KB , 15 pages
This file may not be suitable for users of assistive technology.
Hepatitis A is an infection of the liver caused by hepatitis A virus. The disease is generally mild, but severity tends to increase with age.
Updated details on human normal immunoglobulin (HNIG) administration and Sanofi Pasteur's contact information.
Chapter extensively revised with the latest information.
This chapter has been updated with minor editorial amends. There have been no changes to policies or procedures.
Updated Body text to include link to Green Book update patches on the National Archives website.
First published.
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.

IMAGES
VIDEO
COMMENTS
Details. Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV). Many individuals with a new infection with hepatitis B may have a sub-clinical or a flu-like illness ...
Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV). Many individuals newly infected with hepatitis B may have a sub-clinical or a flu-like illness. Jaundice only occurs in about 10% of younger children and in 30 to 50% of adults. Acute infection may occasionally lead to fulminant hepatic necrosis, which is often fatal.
The Green Book has the latest information on vaccines and vaccination procedures, ... travel and living abroad; Visas and immigration; ... Hepatitis A: the green book, chapter 17.
Hepatitis B is a vaccine-preventable liver disease. Hepatitis B virus is found in the blood and body fluids of infected people. People with hepatitis B virus infection can spread it to others. You can get hepatitis B virus if you. Have sex with an infected partner. Share needles, syringes, or drug preparation equipment with an infected person.
HBV infection primarily affects the liver. Typically, the incubation period for hepatitis B is 90 days (range 60-150 days). Newly acquired acute HBV infections only cause symptoms some of the time, and signs and symptoms vary by age. Most children <5 years of age and immunosuppressed adults are asymptomatic when newly infected, whereas 30% ...
Green Book. 1.1.2 Travel vaccine recommendations . The National Travel Health Network and Centre (NaTHNaC) provides hepatitis B immunisation recommendations for travellers. Risk for travellers is low although certain behaviours or activities put individuals at higher risk, particularly when these occur in areas where hepatitis B is more common.
In the Green Book, hepatitis B for travel is indicated for: "Travellers to areas of high or intermediate prevalence who place themselves at risk when abroad ... Green Book is inappropriate and wasteful of resources, however funded. According to the "Red ook" (paragraph 44.5), 'vaccines [including hepatitis B]..which are supplied ...
Hepatitis B December 2013 Green Book Chapter 18 v20 18 Hepatitis B NOTIFIABLE The disease Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV). Many new infections with hepatitis B are sub-clinical or may have a flu-like illness. Jaundice only occurs in about 10% of younger children and in 30 to 50% of adults.
The recommendations on how to administer hepatitis B vaccines are based on expert opinion in the Department of Health publication Immunisation against infectious disease (The Green Book) Chapter 18: Hepatitis B , Chapter 2: Consent , Chapter 4: Immunisation procedures , and Chapter 8: Vaccine safety and adverse effects , and are also pragmatic ...
The hepatitis B vaccine may also be recommended as a travel vaccine for travel to some parts of the world. ... For more detail, please refer to Chapter 18 of the Green Book. Hepatitis B immunoglobulin. Specific hepatitis B immunoglobulin (HBIG), made from the plasma of people who have been immunised and screened for other infections, such as ...
The 'Green Book' (Immunisation against infectious disease), hepatitis B for travel is indicated for. The former 'Red Book' (Statement of fees and allowances payable to general medical practitioners in England and Wales; not available to download online) advised what services were funded by the NHS:
Hepatitis B (HepB) vaccine for active immunisation of non-immune individuals at high risk of contracting hepatitis B related to travel. This PGD should be used in conjunction with the recommendations in the current British National Formulary (BNF), British National Formulary for Children (BNFC), The Green Book Chapter 18, TRAVAX, NaTHNaC and the
This is an injection which contains antibodies against the virus. It gives short-term protection. You should also start a course of vaccination. The hepatitis B vaccine is very effective at preventing infection if given shortly after contact with hepatitis B. Even if you have had the hepatitis B vaccine and are at risk of infection (for example ...
The combined hepatitis A and hepatitis B vaccine can be prescribed on the NHS because it contains hepatitis A. However, because hepatitis B is not commissioned by the NHS as a travel vaccine, BHCCG does not support the prescribing of this combination vaccine on an NHS prescription for travel. Patients requiring both hepatitis A and B for travel ...
The green book travel chapters. UK Health Security Agency Immunisation against infectious disease, the 'green book' travel chapter updates. The following chapters are the latest updates on travel-related vaccines following discussion at the Joint Committee on Vaccination and Immunisation (JCVI) Travel Sub-Committee and approval by the main JCVI.
hepatitis B virus in accordance with the recommendations given in Chapter 7 and Chapter 18 of Immunisation Against Infectious Disease: 'The Green Book'. Criteria for inclusion Post-exposure Individuals who: • are babies born to hepatitis B infected mothers • have been potentially exposed to hepatitis B infected blood or body fluids
Against Infectious Disease: 'The Green Book'. Criteria for inclusion Individuals over 1 year of age requiring Hepatitis A and Hepatitis B pre-exposure prophylaxis including individuals who: • intend to travel, where hepatitis A and hepatitis B vaccination is currently recommended for travel by NaTHNaC (see the Travel
Hepatitis B: the green book, chapter 18. Updated 9 April 2024. Download CSV 278 KB. This CSV cannot be viewed online.
PHE (2013a) Hepatitis A: the green book, chapter 17. Chapter 17. Public Health England. https://www.gov.uk [Free Full-text] PHE (2013b) Poliomyelitis: the green book, chapter 26. Chapter 26. Public Health England. https://www.gov.uk [Free Full-text] PHE (2013c) Cholera: the green book, chapter 14.
the Green Book, travel guide published (1936-67) during the segregation era in the United States that identified businesses that would accept African American customers. Compiled by Victor Hugo Green (1892-1960), a Black postman who lived in the Harlem section of New York City, the Green Book listed a variety of businesses—from restaurants and hotels to beauty salons and drugstores ...
hepatitis B infected mothers should receive the first dose of vaccine as soon as possible, ideally within 24 hours of birth. • reflect changes to 'The Green Book' recommendations for booster doses • include stability data for Engerix B® • in advice/ follow up section addedthe pre-school
Details. Hepatitis A is an infection of the liver caused by hepatitis A virus. The disease is generally mild, but severity tends to increase with age. Published 20 March 2013. Last updated 15 ...
Hepatitis B Hepatitis B vaccination is recommended for workers who are at risk of injury from blood-contaminated shar p instr uments, or of being deliberatel y injured or bitten by patients. Antibody titres for hepatitis B should be check ed one to four months after the completion of a primary course of vaccine. Such information